Input interpretation
diphenylphosphino ferrocene tetracarbonylchromium
Basic properties
![molar mass | 534.2 g/mol formula | C_26H_19CrFeO_4P empirical formula | C_26O_4Cr_P_Fe_H_19 SMILES identifier | C1=CC=C(C=C1)P(C2=CC=CC=C2)[C-]3C=CC=C3.[CH-]4C=CC=C4.C(=[Cr](=C=O)(=C=O)=C=O)=O.[Fe+2] InChI identifier | InChI=1/C17H14P.C5H5.4CO.Cr.Fe/c1-3-9-15(10-4-1)18(17-13-7-8-14-17)16-11-5-2-6-12-16;1-2-4-5-3-1;4*1-2;;/h1-14H;1-5H;;;;;;/q2*-1;;;;;;+2 InChI key | VDZUQUSLBCZRSX-UHFFFAOYSA-N](../image_source/f4c9be202f240b0dc4fba4b80456aa9b.png)
molar mass | 534.2 g/mol formula | C_26H_19CrFeO_4P empirical formula | C_26O_4Cr_P_Fe_H_19 SMILES identifier | C1=CC=C(C=C1)P(C2=CC=CC=C2)[C-]3C=CC=C3.[CH-]4C=CC=C4.C(=[Cr](=C=O)(=C=O)=C=O)=O.[Fe+2] InChI identifier | InChI=1/C17H14P.C5H5.4CO.Cr.Fe/c1-3-9-15(10-4-1)18(17-13-7-8-14-17)16-11-5-2-6-12-16;1-2-4-5-3-1;4*1-2;;/h1-14H;1-5H;;;;;;/q2*-1;;;;;;+2 InChI key | VDZUQUSLBCZRSX-UHFFFAOYSA-N
Structure diagram
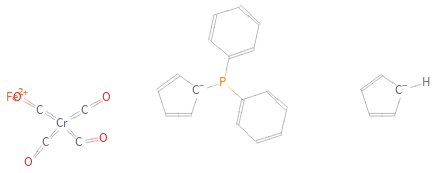
Structure diagram
Quantitative molecular descriptors
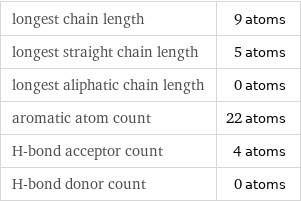
longest chain length | 9 atoms longest straight chain length | 5 atoms longest aliphatic chain length | 0 atoms aromatic atom count | 22 atoms H-bond acceptor count | 4 atoms H-bond donor count | 0 atoms
Elemental composition
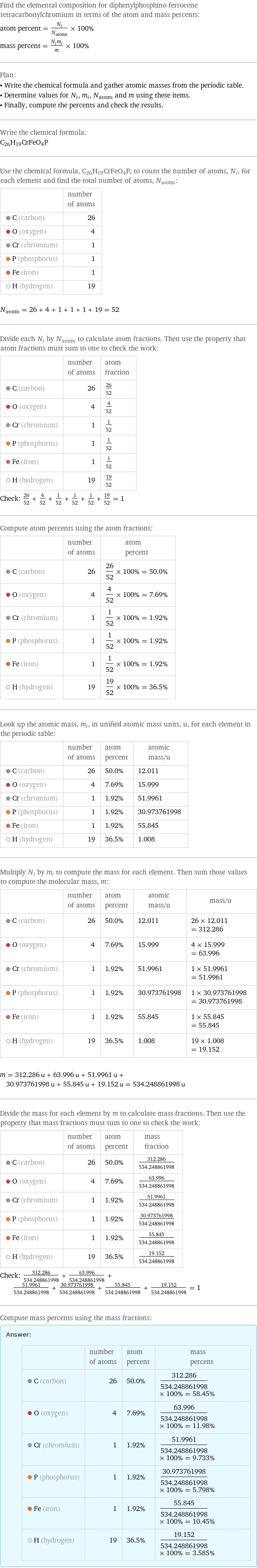
Find the elemental composition for diphenylphosphino ferrocene tetracarbonylchromium in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: C_26H_19CrFeO_4P Use the chemical formula, C_26H_19CrFeO_4P, to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms: | number of atoms C (carbon) | 26 O (oxygen) | 4 Cr (chromium) | 1 P (phosphorus) | 1 Fe (iron) | 1 H (hydrogen) | 19 N_atoms = 26 + 4 + 1 + 1 + 1 + 19 = 52 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction C (carbon) | 26 | 26/52 O (oxygen) | 4 | 4/52 Cr (chromium) | 1 | 1/52 P (phosphorus) | 1 | 1/52 Fe (iron) | 1 | 1/52 H (hydrogen) | 19 | 19/52 Check: 26/52 + 4/52 + 1/52 + 1/52 + 1/52 + 19/52 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent C (carbon) | 26 | 26/52 × 100% = 50.0% O (oxygen) | 4 | 4/52 × 100% = 7.69% Cr (chromium) | 1 | 1/52 × 100% = 1.92% P (phosphorus) | 1 | 1/52 × 100% = 1.92% Fe (iron) | 1 | 1/52 × 100% = 1.92% H (hydrogen) | 19 | 19/52 × 100% = 36.5% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u C (carbon) | 26 | 50.0% | 12.011 O (oxygen) | 4 | 7.69% | 15.999 Cr (chromium) | 1 | 1.92% | 51.9961 P (phosphorus) | 1 | 1.92% | 30.973761998 Fe (iron) | 1 | 1.92% | 55.845 H (hydrogen) | 19 | 36.5% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u C (carbon) | 26 | 50.0% | 12.011 | 26 × 12.011 = 312.286 O (oxygen) | 4 | 7.69% | 15.999 | 4 × 15.999 = 63.996 Cr (chromium) | 1 | 1.92% | 51.9961 | 1 × 51.9961 = 51.9961 P (phosphorus) | 1 | 1.92% | 30.973761998 | 1 × 30.973761998 = 30.973761998 Fe (iron) | 1 | 1.92% | 55.845 | 1 × 55.845 = 55.845 H (hydrogen) | 19 | 36.5% | 1.008 | 19 × 1.008 = 19.152 m = 312.286 u + 63.996 u + 51.9961 u + 30.973761998 u + 55.845 u + 19.152 u = 534.248861998 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction C (carbon) | 26 | 50.0% | 312.286/534.248861998 O (oxygen) | 4 | 7.69% | 63.996/534.248861998 Cr (chromium) | 1 | 1.92% | 51.9961/534.248861998 P (phosphorus) | 1 | 1.92% | 30.973761998/534.248861998 Fe (iron) | 1 | 1.92% | 55.845/534.248861998 H (hydrogen) | 19 | 36.5% | 19.152/534.248861998 Check: 312.286/534.248861998 + 63.996/534.248861998 + 51.9961/534.248861998 + 30.973761998/534.248861998 + 55.845/534.248861998 + 19.152/534.248861998 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent C (carbon) | 26 | 50.0% | 312.286/534.248861998 × 100% = 58.45% O (oxygen) | 4 | 7.69% | 63.996/534.248861998 × 100% = 11.98% Cr (chromium) | 1 | 1.92% | 51.9961/534.248861998 × 100% = 9.733% P (phosphorus) | 1 | 1.92% | 30.973761998/534.248861998 × 100% = 5.798% Fe (iron) | 1 | 1.92% | 55.845/534.248861998 × 100% = 10.45% H (hydrogen) | 19 | 36.5% | 19.152/534.248861998 × 100% = 3.585%
Topological indices
vertex count | 52 edge count | 52 Schultz index | Wiener index | Hosoya index | Balaban index |