Input interpretation

solid elements (at STP) | atomic volume
Summary

median | 1.56×10^7 pm^3 highest | 7.4×10^7 pm^3 (cesium) lowest | 1.4×10^6 pm^3 (carbon) distribution | | (based on 81 values; 5 unavailable)
Entities with missing values
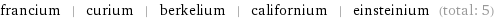
francium | curium | berkelium | californium | einsteinium (total: 5)
Plot
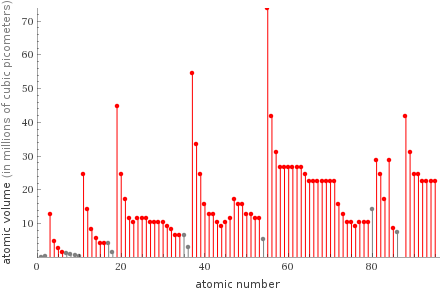
Plot
Periodic table location
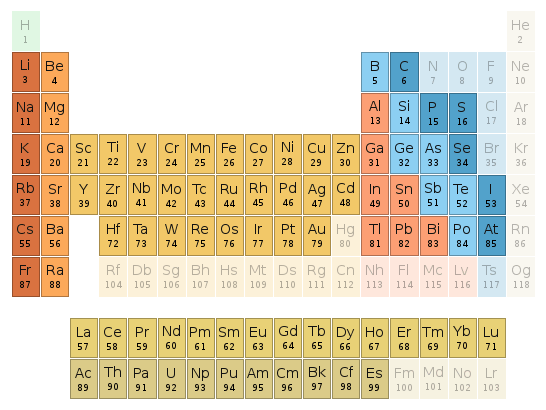
Periodic table location
Atomic radii properties
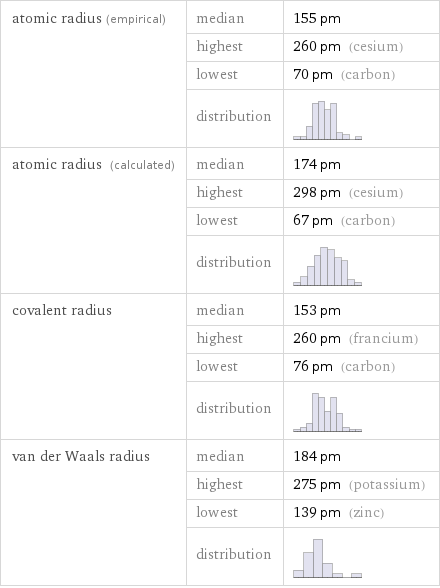
atomic radius (empirical) | median | 155 pm | highest | 260 pm (cesium) | lowest | 70 pm (carbon) | distribution | atomic radius (calculated) | median | 174 pm | highest | 298 pm (cesium) | lowest | 67 pm (carbon) | distribution | covalent radius | median | 153 pm | highest | 260 pm (francium) | lowest | 76 pm (carbon) | distribution | van der Waals radius | median | 184 pm | highest | 275 pm (potassium) | lowest | 139 pm (zinc) | distribution |
Units

Distribution plots
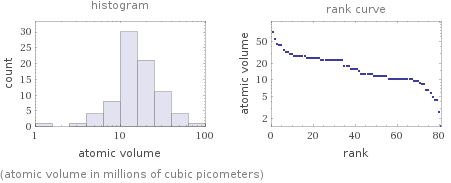
(atomic volume in millions of cubic picometers)
Atomic volume rankings
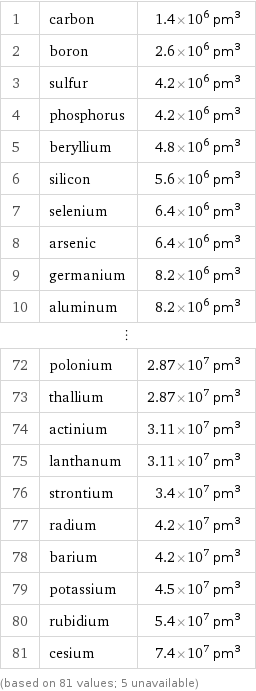
1 | carbon | 1.4×10^6 pm^3 2 | boron | 2.6×10^6 pm^3 3 | sulfur | 4.2×10^6 pm^3 4 | phosphorus | 4.2×10^6 pm^3 5 | beryllium | 4.8×10^6 pm^3 6 | silicon | 5.6×10^6 pm^3 7 | selenium | 6.4×10^6 pm^3 8 | arsenic | 6.4×10^6 pm^3 9 | germanium | 8.2×10^6 pm^3 10 | aluminum | 8.2×10^6 pm^3 ⋮ | | 72 | polonium | 2.87×10^7 pm^3 73 | thallium | 2.87×10^7 pm^3 74 | actinium | 3.11×10^7 pm^3 75 | lanthanum | 3.11×10^7 pm^3 76 | strontium | 3.4×10^7 pm^3 77 | radium | 4.2×10^7 pm^3 78 | barium | 4.2×10^7 pm^3 79 | potassium | 4.5×10^7 pm^3 80 | rubidium | 5.4×10^7 pm^3 81 | cesium | 7.4×10^7 pm^3 (based on 81 values; 5 unavailable)
Unit conversions for median atomic volume 1.56×10^7 pm^3
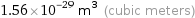
1.56×10^-29 m^3 (cubic meters)
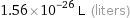
1.56×10^-26 L (liters)
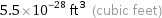
5.5×10^-28 ft^3 (cubic feet)
Corresponding quantities

Radius r of a sphere from V = 4πr^3/3: | 155 pm (picometers) | 6.1×10^-9 inches

Edge length a of a cube from V = a^3: | 250 pm (picometers) | 9.8×10^-9 inches