Input interpretation
cobalt monoxide
Chemical names and formulas
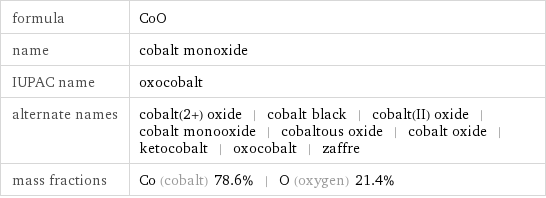
formula | CoO name | cobalt monoxide IUPAC name | oxocobalt alternate names | cobalt(2+) oxide | cobalt black | cobalt(II) oxide | cobalt monooxide | cobaltous oxide | cobalt oxide | ketocobalt | oxocobalt | zaffre mass fractions | Co (cobalt) 78.6% | O (oxygen) 21.4%
Structure diagram
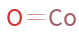
Structure diagram
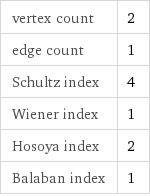
vertex count | 2 edge count | 1 Schultz index | 4 Wiener index | 1 Hosoya index | 2 Balaban index | 1
Basic properties
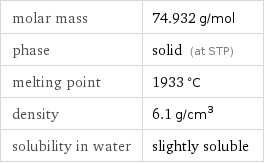
molar mass | 74.932 g/mol phase | solid (at STP) melting point | 1933 °C density | 6.1 g/cm^3 solubility in water | slightly soluble
Units

Solid properties (at STP)

density | 6.1 g/cm^3
Units

Thermodynamic properties
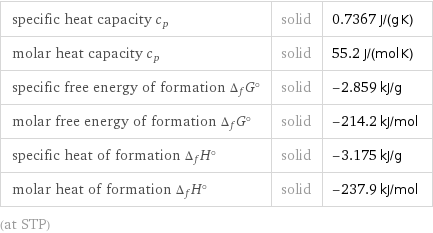
specific heat capacity c_p | solid | 0.7367 J/(g K) molar heat capacity c_p | solid | 55.2 J/(mol K) specific free energy of formation Δ_fG° | solid | -2.859 kJ/g molar free energy of formation Δ_fG° | solid | -214.2 kJ/mol specific heat of formation Δ_fH° | solid | -3.175 kJ/g molar heat of formation Δ_fH° | solid | -237.9 kJ/mol (at STP)
Chemical identifiers
![CAS number | 1307-96-6 PubChem CID number | 14786 PubChem SID number | 24861123 SMILES identifier | O=[Co] InChI identifier | InChI=1/Co.O/rCoO/c1-2 RTECS number | GG2800000 MDL number | MFCD00016031](../image_source/802ba95614c6bb0b732ec8b40c45f174.png)
CAS number | 1307-96-6 PubChem CID number | 14786 PubChem SID number | 24861123 SMILES identifier | O=[Co] InChI identifier | InChI=1/Co.O/rCoO/c1-2 RTECS number | GG2800000 MDL number | MFCD00016031
Toxicity properties
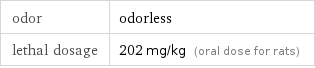
odor | odorless lethal dosage | 202 mg/kg (oral dose for rats)

probable lethal dose for man | 30 mL (milliliters) RTECS classes | tumorigen