Input interpretation
barium chlorate | acetyl fluoride
Chemical names and formulas
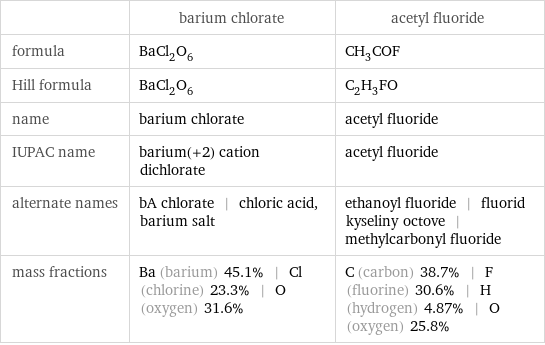
| barium chlorate | acetyl fluoride formula | BaCl_2O_6 | CH_3COF Hill formula | BaCl_2O_6 | C_2H_3FO name | barium chlorate | acetyl fluoride IUPAC name | barium(+2) cation dichlorate | acetyl fluoride alternate names | bA chlorate | chloric acid, barium salt | ethanoyl fluoride | fluorid kyseliny octove | methylcarbonyl fluoride mass fractions | Ba (barium) 45.1% | Cl (chlorine) 23.3% | O (oxygen) 31.6% | C (carbon) 38.7% | F (fluorine) 30.6% | H (hydrogen) 4.87% | O (oxygen) 25.8%
Structure diagrams
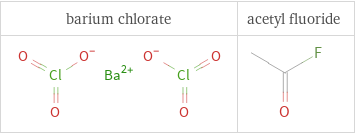
Structure diagrams
3D structure
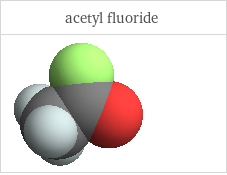
3D structure
Basic properties
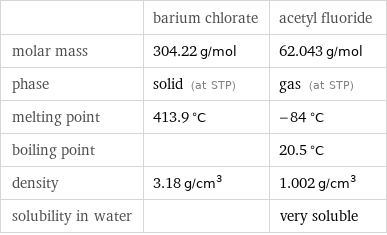
| barium chlorate | acetyl fluoride molar mass | 304.22 g/mol | 62.043 g/mol phase | solid (at STP) | gas (at STP) melting point | 413.9 °C | -84 °C boiling point | | 20.5 °C density | 3.18 g/cm^3 | 1.002 g/cm^3 solubility in water | | very soluble
Units

Gas properties
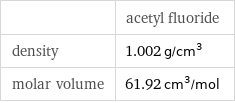
| acetyl fluoride density | 1.002 g/cm^3 molar volume | 61.92 cm^3/mol
Units
