Input interpretation

solid compounds | relative molecular mass
Summary
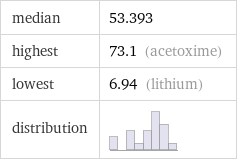
median | 53.393 highest | 73.1 (acetoxime) lowest | 6.94 (lithium) distribution |
Distribution plots
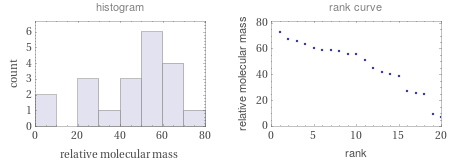
Relative molecular mass rankings
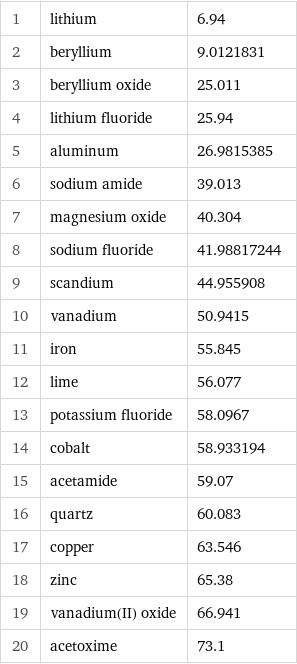
1 | lithium | 6.94 2 | beryllium | 9.0121831 3 | beryllium oxide | 25.011 4 | lithium fluoride | 25.94 5 | aluminum | 26.9815385 6 | sodium amide | 39.013 7 | magnesium oxide | 40.304 8 | sodium fluoride | 41.98817244 9 | scandium | 44.955908 10 | vanadium | 50.9415 11 | iron | 55.845 12 | lime | 56.077 13 | potassium fluoride | 58.0967 14 | cobalt | 58.933194 15 | acetamide | 59.07 16 | quartz | 60.083 17 | copper | 63.546 18 | zinc | 65.38 19 | vanadium(II) oxide | 66.941 20 | acetoxime | 73.1
Comparisons for median relative molecular mass 53.393
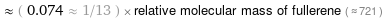
≈ ( 0.074 ≈ 1/13 ) × relative molecular mass of fullerene ( ≈ 721 )

≈ 0.27 × relative molecular mass of caffeine ( ≈ 194 )
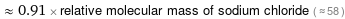
≈ 0.91 × relative molecular mass of sodium chloride ( ≈ 58 )
Corresponding quantities
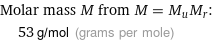
Molar mass M from M = M_uM_r: | 53 g/mol (grams per mole)

Molecular mass m from m = M_rM_u/N_A: | 8.9×10^-23 grams | 8.9×10^-26 kg (kilograms)