Input interpretation
magnesium chloride
Chemical names and formulas
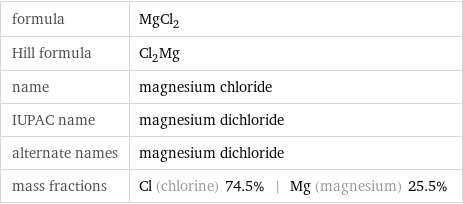
formula | MgCl_2 Hill formula | Cl_2Mg name | magnesium chloride IUPAC name | magnesium dichloride alternate names | magnesium dichloride mass fractions | Cl (chlorine) 74.5% | Mg (magnesium) 25.5%
Structure diagram
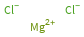
Structure diagram
Basic properties
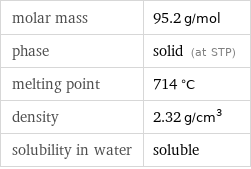
molar mass | 95.2 g/mol phase | solid (at STP) melting point | 714 °C density | 2.32 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)

density | 2.32 g/cm^3
Units

Thermodynamic properties
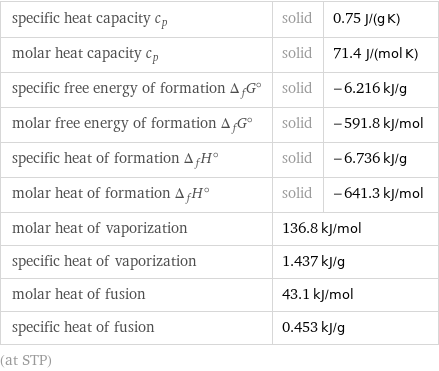
specific heat capacity c_p | solid | 0.75 J/(g K) molar heat capacity c_p | solid | 71.4 J/(mol K) specific free energy of formation Δ_fG° | solid | -6.216 kJ/g molar free energy of formation Δ_fG° | solid | -591.8 kJ/mol specific heat of formation Δ_fH° | solid | -6.736 kJ/g molar heat of formation Δ_fH° | solid | -641.3 kJ/mol molar heat of vaporization | 136.8 kJ/mol | specific heat of vaporization | 1.437 kJ/g | molar heat of fusion | 43.1 kJ/mol | specific heat of fusion | 0.453 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7786-30-3 PubChem CID number | 24584 PubChem SID number | 24852507 SMILES identifier | [Mg+2].[Cl-].[Cl-] InChI identifier | InChI=1/2ClH.Mg/h2*1H;/q;;+2/p-2/f2Cl.Mg/h2*1h;/q2*-1;m RTECS number | OM2800000 MDL number | MFCD00011106](../image_source/6c27678c56315660c8423b5f9656c8b8.png)
CAS number | 7786-30-3 PubChem CID number | 24584 PubChem SID number | 24852507 SMILES identifier | [Mg+2].[Cl-].[Cl-] InChI identifier | InChI=1/2ClH.Mg/h2*1H;/q;;+2/p-2/f2Cl.Mg/h2*1h;/q2*-1;m RTECS number | OM2800000 MDL number | MFCD00011106
Ion equivalents

Mg^(2+) (magnesium cation) | 1 Cl^- (chloride anion) | 2