Input interpretation
sodium hexachloroiridate(IV) hexahydrate | fluoboric acid
Chemical names and formulas
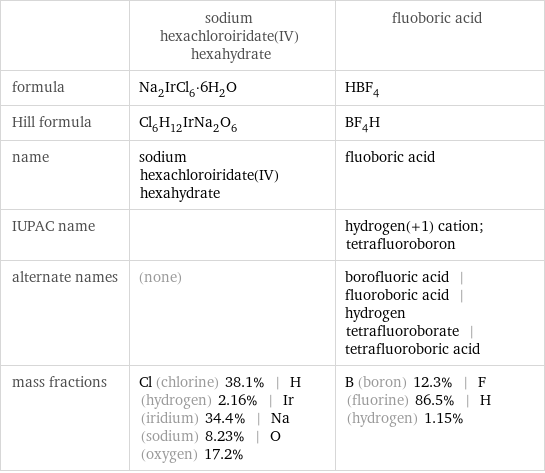
| sodium hexachloroiridate(IV) hexahydrate | fluoboric acid formula | Na_2IrCl_6·6H_2O | HBF_4 Hill formula | Cl_6H_12IrNa_2O_6 | BF_4H name | sodium hexachloroiridate(IV) hexahydrate | fluoboric acid IUPAC name | | hydrogen(+1) cation; tetrafluoroboron alternate names | (none) | borofluoric acid | fluoroboric acid | hydrogen tetrafluoroborate | tetrafluoroboric acid mass fractions | Cl (chlorine) 38.1% | H (hydrogen) 2.16% | Ir (iridium) 34.4% | Na (sodium) 8.23% | O (oxygen) 17.2% | B (boron) 12.3% | F (fluorine) 86.5% | H (hydrogen) 1.15%
Structure diagrams
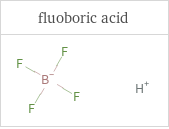
Structure diagrams
Basic properties

| fluoboric acid molar mass | 87.81 g/mol phase | liquid (at STP) melting point | -90 °C boiling point | 130 °C density | 1.41 g/cm^3 solubility in water | miscible
Units
