Input interpretation
silver iodate | hydrogen peroxide
Chemical names and formulas
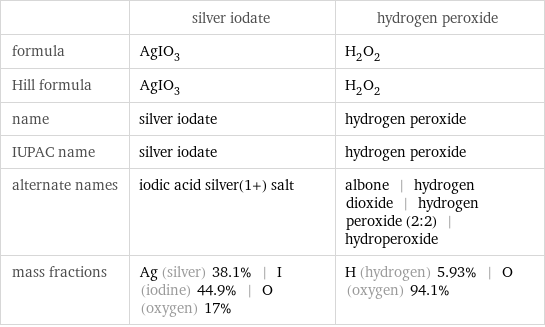
| silver iodate | hydrogen peroxide formula | AgIO_3 | H_2O_2 Hill formula | AgIO_3 | H_2O_2 name | silver iodate | hydrogen peroxide IUPAC name | silver iodate | hydrogen peroxide alternate names | iodic acid silver(1+) salt | albone | hydrogen dioxide | hydrogen peroxide (2:2) | hydroperoxide mass fractions | Ag (silver) 38.1% | I (iodine) 44.9% | O (oxygen) 17% | H (hydrogen) 5.93% | O (oxygen) 94.1%
Structure diagrams
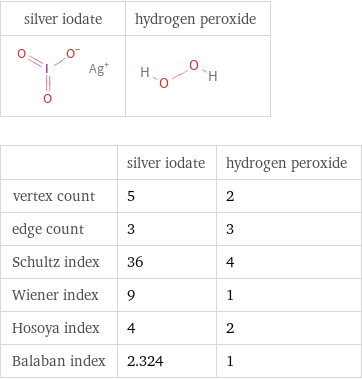
| silver iodate | hydrogen peroxide vertex count | 5 | 2 edge count | 3 | 3 Schultz index | 36 | 4 Wiener index | 9 | 1 Hosoya index | 4 | 2 Balaban index | 2.324 | 1
3D structure
3D structure
Basic properties
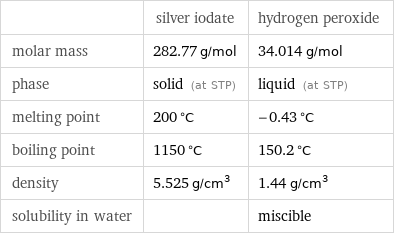
| silver iodate | hydrogen peroxide molar mass | 282.77 g/mol | 34.014 g/mol phase | solid (at STP) | liquid (at STP) melting point | 200 °C | -0.43 °C boiling point | 1150 °C | 150.2 °C density | 5.525 g/cm^3 | 1.44 g/cm^3 solubility in water | | miscible
Units

Liquid properties
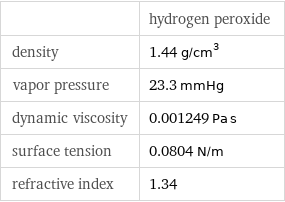
| hydrogen peroxide density | 1.44 g/cm^3 vapor pressure | 23.3 mmHg dynamic viscosity | 0.001249 Pa s surface tension | 0.0804 N/m refractive index | 1.34
Units
Thermodynamic properties
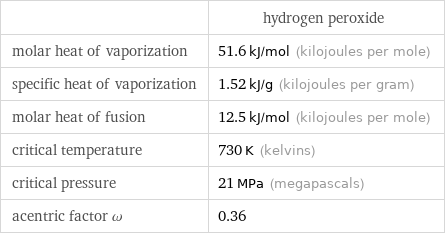
| hydrogen peroxide molar heat of vaporization | 51.6 kJ/mol (kilojoules per mole) specific heat of vaporization | 1.52 kJ/g (kilojoules per gram) molar heat of fusion | 12.5 kJ/mol (kilojoules per mole) critical temperature | 730 K (kelvins) critical pressure | 21 MPa (megapascals) acentric factor ω | 0.36
Solid properties
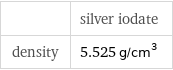
| silver iodate density | 5.525 g/cm^3
Units
