Input interpretation
iodic acid | sodium fluoride
Chemical names and formulas
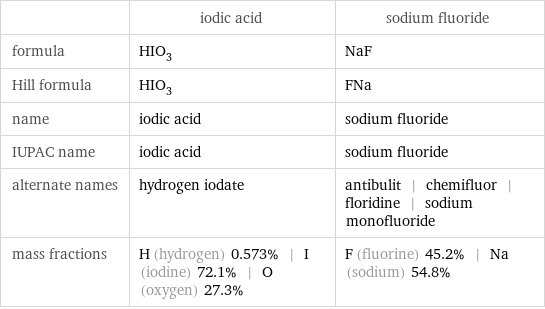
| iodic acid | sodium fluoride formula | HIO_3 | NaF Hill formula | HIO_3 | FNa name | iodic acid | sodium fluoride IUPAC name | iodic acid | sodium fluoride alternate names | hydrogen iodate | antibulit | chemifluor | floridine | sodium monofluoride mass fractions | H (hydrogen) 0.573% | I (iodine) 72.1% | O (oxygen) 27.3% | F (fluorine) 45.2% | Na (sodium) 54.8%
Structure diagrams
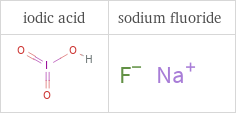
Structure diagrams
Basic properties
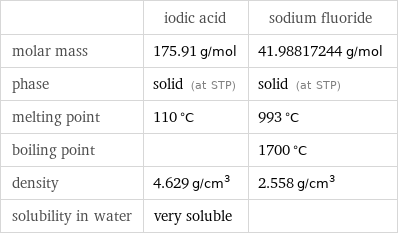
| iodic acid | sodium fluoride molar mass | 175.91 g/mol | 41.98817244 g/mol phase | solid (at STP) | solid (at STP) melting point | 110 °C | 993 °C boiling point | | 1700 °C density | 4.629 g/cm^3 | 2.558 g/cm^3 solubility in water | very soluble |
Units

Solid properties (at STP)
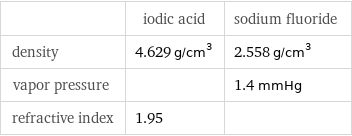
| iodic acid | sodium fluoride density | 4.629 g/cm^3 | 2.558 g/cm^3 vapor pressure | | 1.4 mmHg refractive index | 1.95 |
Units

Chemical identifiers
![| iodic acid | sodium fluoride CAS number | 7782-68-5 | 7681-49-4 PubChem CID number | 24345 | 5235 PubChem SID number | | 24852884 SMILES identifier | OI(=O)=O | [F-].[Na+]](../image_source/32b213a3e8e5b1fdaecb68da06c08d62.png)
| iodic acid | sodium fluoride CAS number | 7782-68-5 | 7681-49-4 PubChem CID number | 24345 | 5235 PubChem SID number | | 24852884 SMILES identifier | OI(=O)=O | [F-].[Na+]