Input interpretation
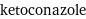
ketoconazole
Chemical names and formulas
![formula | C_26H_28Cl_2N_4O_4 name | ketoconazole IUPAC name | 1-[4-[4-[[(2S, 4S)-2-(2, 4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1, 3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone alternate names | fungoral | nizoral | nizral mass fractions | C (carbon) 58.8% | Cl (chlorine) 13.3% | H (hydrogen) 5.31% | N (nitrogen) 10.5% | O (oxygen) 12%](../image_source/6322fe0e1a8f245db461c135e3090327.png)
formula | C_26H_28Cl_2N_4O_4 name | ketoconazole IUPAC name | 1-[4-[4-[[(2S, 4S)-2-(2, 4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1, 3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone alternate names | fungoral | nizoral | nizral mass fractions | C (carbon) 58.8% | Cl (chlorine) 13.3% | H (hydrogen) 5.31% | N (nitrogen) 10.5% | O (oxygen) 12%
Lewis structure
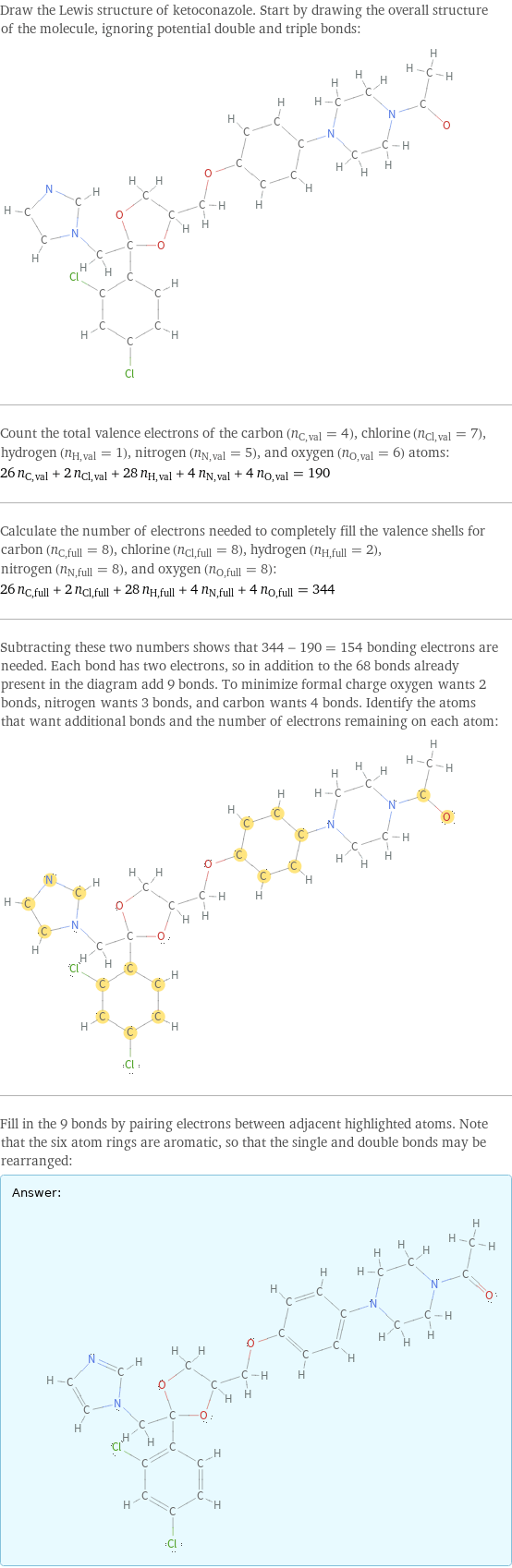
Draw the Lewis structure of ketoconazole. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 26 n_C, val + 2 n_Cl, val + 28 n_H, val + 4 n_N, val + 4 n_O, val = 190 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 26 n_C, full + 2 n_Cl, full + 28 n_H, full + 4 n_N, full + 4 n_O, full = 344 Subtracting these two numbers shows that 344 - 190 = 154 bonding electrons are needed. Each bond has two electrons, so in addition to the 68 bonds already present in the diagram add 9 bonds. To minimize formal charge oxygen wants 2 bonds, nitrogen wants 3 bonds, and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 9 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom rings are aromatic, so that the single and double bonds may be rearranged: Answer: | |
3D structure
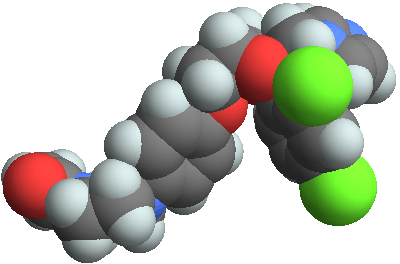
3D structure
Basic properties
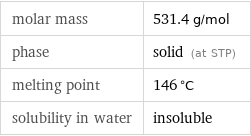
molar mass | 531.4 g/mol phase | solid (at STP) melting point | 146 °C solubility in water | insoluble
Units

Hydrophobicity and permeability properties

experimental LogP hydrophobicity | 4 predicted LogP hydrophobicity | 4.31 predicted LogS | -4.76
Drug interactions

acenocoumarol | alfentanil | alfuzosin | almotriptan | alprazolam | aluminum | amitriptyline | anisindione | aprepitant | aripiprazole | astemizole | atorvastatin | bismuth | bosentan | budesonide | buspirone | calcium | carbamazepine | cerivastatin | chlordiazepoxide | ... (total: 102)
Basic drug properties

approval status | approved | investigational | small molecule drug categories | antifungal agent | antifungal dosage forms | topical: cream | topical: shampoo | oral: tablet

brand names | extina | fungarest | fungoral | ketoderm | ketoisdin | ketozole | nizoral | nizoral cream | nizoral shampoo | nizoral a-D | nizoral a-D shampoo | orifungal | orifungal M | panfungol | sebazole
Chemical identifiers
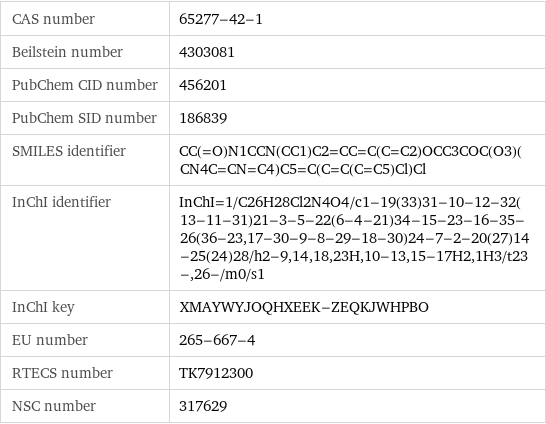
CAS number | 65277-42-1 Beilstein number | 4303081 PubChem CID number | 456201 PubChem SID number | 186839 SMILES identifier | CC(=O)N1CCN(CC1)C2=CC=C(C=C2)OCC3COC(O3)(CN4C=CN=C4)C5=C(C=C(C=C5)Cl)Cl InChI identifier | InChI=1/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23, 17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9, 14, 18, 23H, 10-13, 15-17H2, 1H3/t23-, 26-/m0/s1 InChI key | XMAYWYJOQHXEEK-ZEQKJWHPBO EU number | 265-667-4 RTECS number | TK7912300 NSC number | 317629
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | drug | mutagen | reproductive effector | human data