Input interpretation
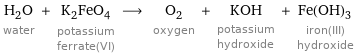
H_2O water + K_2FeO_4 potassium ferrate(VI) ⟶ O_2 oxygen + KOH potassium hydroxide + Fe(OH)_3 iron(III) hydroxide
Sample reactions
(data not available)
Chemical names and formulas
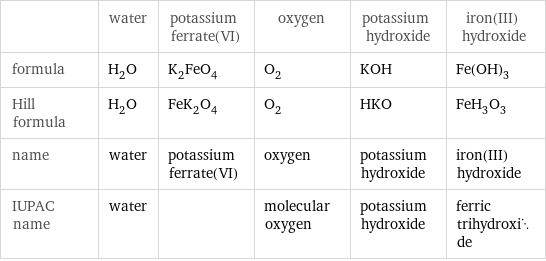
| water | potassium ferrate(VI) | oxygen | potassium hydroxide | iron(III) hydroxide formula | H_2O | K_2FeO_4 | O_2 | KOH | Fe(OH)_3 Hill formula | H_2O | FeK_2O_4 | O_2 | HKO | FeH_3O_3 name | water | potassium ferrate(VI) | oxygen | potassium hydroxide | iron(III) hydroxide IUPAC name | water | | molecular oxygen | potassium hydroxide | ferric trihydroxide