Input interpretation
potassium phosphinate | ammonium ferricyanide trihydrate
Chemical names and formulas
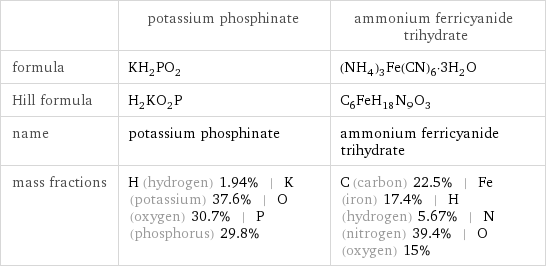
| potassium phosphinate | ammonium ferricyanide trihydrate formula | KH_2PO_2 | (NH_4)_3Fe(CN)_6·3H_2O Hill formula | H_2KO_2P | C_6FeH_18N_9O_3 name | potassium phosphinate | ammonium ferricyanide trihydrate mass fractions | H (hydrogen) 1.94% | K (potassium) 37.6% | O (oxygen) 30.7% | P (phosphorus) 29.8% | C (carbon) 22.5% | Fe (iron) 17.4% | H (hydrogen) 5.67% | N (nitrogen) 39.4% | O (oxygen) 15%
Structure diagrams
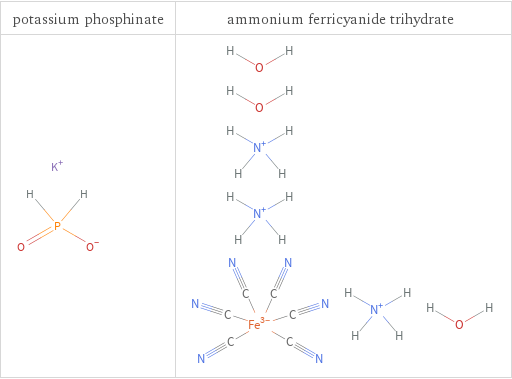
Structure diagrams
Basic properties

| potassium phosphinate | ammonium ferricyanide trihydrate molar mass | 104.09 g/mol | 320.12 g/mol solubility in water | soluble | soluble
Units
