Input interpretation
lithium chloride | sodium bromide
Chemical names and formulas
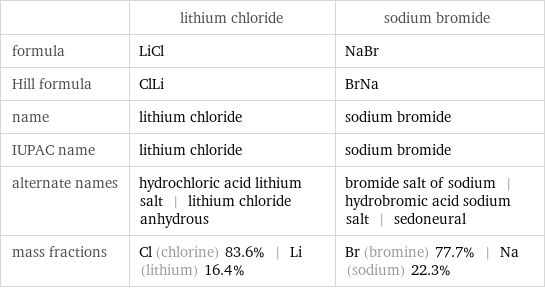
| lithium chloride | sodium bromide formula | LiCl | NaBr Hill formula | ClLi | BrNa name | lithium chloride | sodium bromide IUPAC name | lithium chloride | sodium bromide alternate names | hydrochloric acid lithium salt | lithium chloride anhydrous | bromide salt of sodium | hydrobromic acid sodium salt | sedoneural mass fractions | Cl (chlorine) 83.6% | Li (lithium) 16.4% | Br (bromine) 77.7% | Na (sodium) 22.3%
Structure diagrams

Structure diagrams
Basic properties
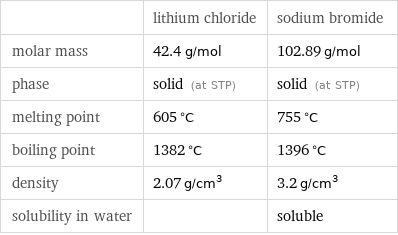
| lithium chloride | sodium bromide molar mass | 42.4 g/mol | 102.89 g/mol phase | solid (at STP) | solid (at STP) melting point | 605 °C | 755 °C boiling point | 1382 °C | 1396 °C density | 2.07 g/cm^3 | 3.2 g/cm^3 solubility in water | | soluble
Units

Thermodynamic properties
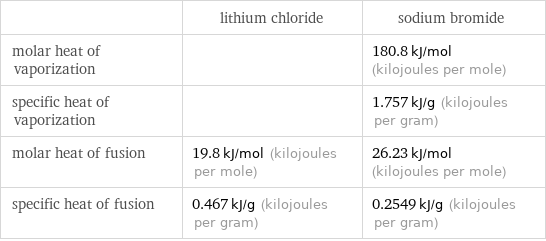
| lithium chloride | sodium bromide molar heat of vaporization | | 180.8 kJ/mol (kilojoules per mole) specific heat of vaporization | | 1.757 kJ/g (kilojoules per gram) molar heat of fusion | 19.8 kJ/mol (kilojoules per mole) | 26.23 kJ/mol (kilojoules per mole) specific heat of fusion | 0.467 kJ/g (kilojoules per gram) | 0.2549 kJ/g (kilojoules per gram)
Solid properties (at STP)

| lithium chloride | sodium bromide density | 2.07 g/cm^3 | 3.2 g/cm^3 vapor pressure | 360 mmHg | 0.9998 mmHg refractive index | | 1.6459
Units

Chemical identifiers
![| lithium chloride | sodium bromide CAS number | 7447-41-8 | 7647-15-6 PubChem CID number | 433294 | 253881 PubChem SID number | 24852187 | 24853756 SMILES identifier | [Li+].[Cl-] | [Na+].[Br-] InChI identifier | InChI=1/ClH.Li/h1H;/q;+1/p-1/fCl.Li/h1h;/q-1;m | InChI=1/BrH.Na/h1H;/q;+1/p-1/fBr.Na/h1h;/q-1;m RTECS number | OJ5950000 | VZ3150000 MDL number | MFCD00011078 | MFCD00003475](../image_source/090fcb7155709bd68c9d15705eaba604.png)
| lithium chloride | sodium bromide CAS number | 7447-41-8 | 7647-15-6 PubChem CID number | 433294 | 253881 PubChem SID number | 24852187 | 24853756 SMILES identifier | [Li+].[Cl-] | [Na+].[Br-] InChI identifier | InChI=1/ClH.Li/h1H;/q;+1/p-1/fCl.Li/h1h;/q-1;m | InChI=1/BrH.Na/h1H;/q;+1/p-1/fBr.Na/h1h;/q-1;m RTECS number | OJ5950000 | VZ3150000 MDL number | MFCD00011078 | MFCD00003475
NFPA label
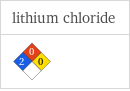
NFPA label
| lithium chloride NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Safety properties

| sodium bromide flash point | 800 °C