Input interpretation
chlorine (chemical element) | sulfur (chemical element) | polonium (chemical element) | boron (chemical element) | lead (chemical element)
Periodic table location
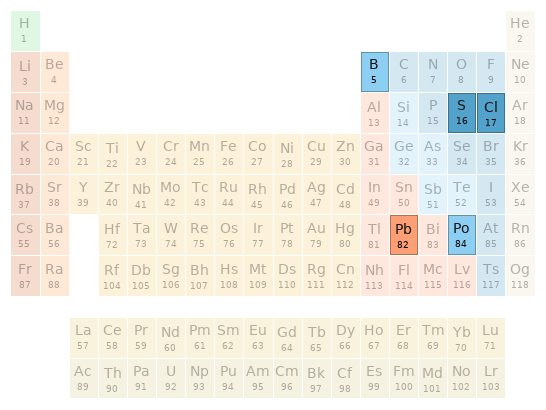
Periodic table location
Images
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration chlorine | Cl | 17 | [Ne]3s^23p^5 sulfur | S | 16 | [Ne]3s^23p^4 polonium | Po | 84 | [Xe]6s^24f^145d^106p^4 boron | B | 5 | [He]2s^22p^1 lead | Pb | 82 | [Xe]6s^24f^145d^106p^2 | Aufbau diagram | block | group chlorine | 3p 3s | p | 17 sulfur | 3p 3s | p | 16 polonium | 6p 5d 4f 6s | p | 16 boron | 2p 2s | p | 13 lead | 6p 5d 4f 6s | p | 14 | period | atomic mass | half-life chlorine | 3 | 35.45 u | (stable) sulfur | 3 | 32.06 u | (stable) polonium | 6 | 209 u | 102 yr boron | 2 | 10.81 u | (stable) lead | 6 | 207.2 u | (stable)](../image_source/6abeca3c5e18fae73349350143655989.png)
| atomic symbol | atomic number | short electronic configuration chlorine | Cl | 17 | [Ne]3s^23p^5 sulfur | S | 16 | [Ne]3s^23p^4 polonium | Po | 84 | [Xe]6s^24f^145d^106p^4 boron | B | 5 | [He]2s^22p^1 lead | Pb | 82 | [Xe]6s^24f^145d^106p^2 | Aufbau diagram | block | group chlorine | 3p 3s | p | 17 sulfur | 3p 3s | p | 16 polonium | 6p 5d 4f 6s | p | 16 boron | 2p 2s | p | 13 lead | 6p 5d 4f 6s | p | 14 | period | atomic mass | half-life chlorine | 3 | 35.45 u | (stable) sulfur | 3 | 32.06 u | (stable) polonium | 6 | 209 u | 102 yr boron | 2 | 10.81 u | (stable) lead | 6 | 207.2 u | (stable)
Thermodynamic properties
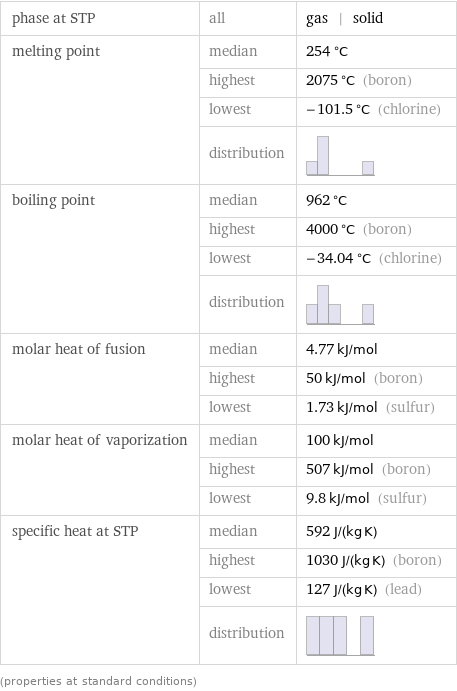
phase at STP | all | gas | solid melting point | median | 254 °C | highest | 2075 °C (boron) | lowest | -101.5 °C (chlorine) | distribution | boiling point | median | 962 °C | highest | 4000 °C (boron) | lowest | -34.04 °C (chlorine) | distribution | molar heat of fusion | median | 4.77 kJ/mol | highest | 50 kJ/mol (boron) | lowest | 1.73 kJ/mol (sulfur) molar heat of vaporization | median | 100 kJ/mol | highest | 507 kJ/mol (boron) | lowest | 9.8 kJ/mol (sulfur) specific heat at STP | median | 592 J/(kg K) | highest | 1030 J/(kg K) (boron) | lowest | 127 J/(kg K) (lead) | distribution | (properties at standard conditions)
Material properties
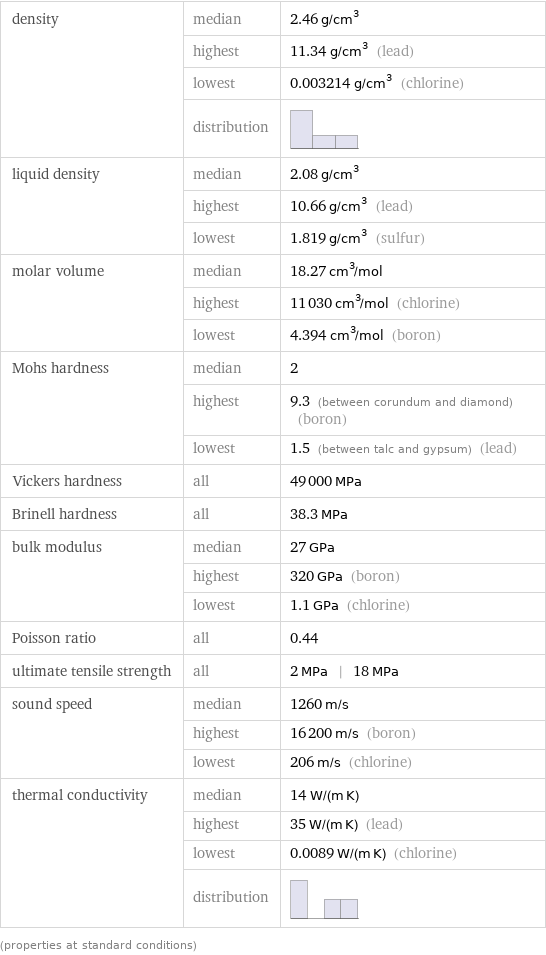
density | median | 2.46 g/cm^3 | highest | 11.34 g/cm^3 (lead) | lowest | 0.003214 g/cm^3 (chlorine) | distribution | liquid density | median | 2.08 g/cm^3 | highest | 10.66 g/cm^3 (lead) | lowest | 1.819 g/cm^3 (sulfur) molar volume | median | 18.27 cm^3/mol | highest | 11030 cm^3/mol (chlorine) | lowest | 4.394 cm^3/mol (boron) Mohs hardness | median | 2 | highest | 9.3 (between corundum and diamond) (boron) | lowest | 1.5 (between talc and gypsum) (lead) Vickers hardness | all | 49000 MPa Brinell hardness | all | 38.3 MPa bulk modulus | median | 27 GPa | highest | 320 GPa (boron) | lowest | 1.1 GPa (chlorine) Poisson ratio | all | 0.44 ultimate tensile strength | all | 2 MPa | 18 MPa sound speed | median | 1260 m/s | highest | 16200 m/s (boron) | lowest | 206 m/s (chlorine) thermal conductivity | median | 14 W/(m K) | highest | 35 W/(m K) (lead) | lowest | 0.0089 W/(m K) (chlorine) | distribution | (properties at standard conditions)
Electromagnetic properties
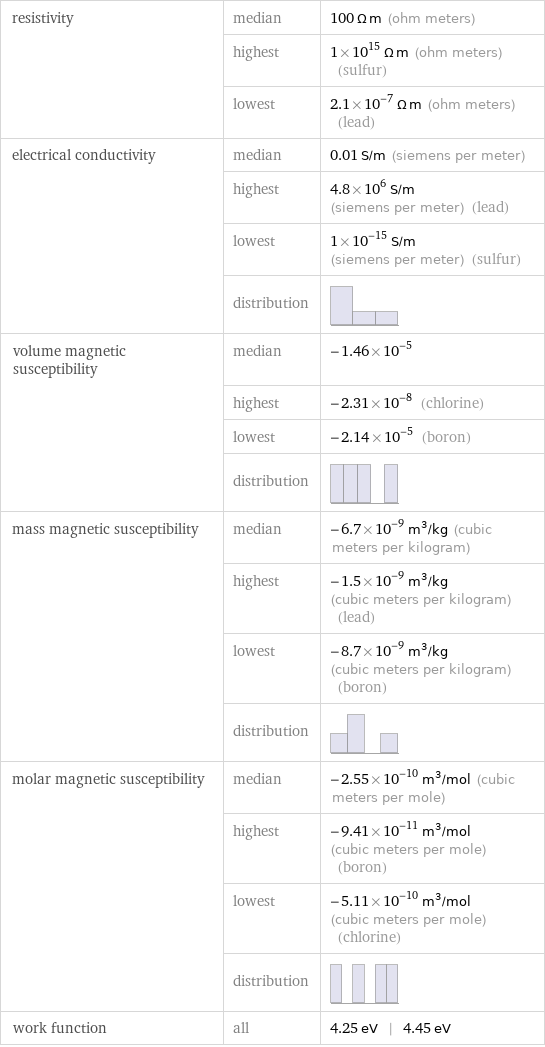
resistivity | median | 100 Ω m (ohm meters) | highest | 1×10^15 Ω m (ohm meters) (sulfur) | lowest | 2.1×10^-7 Ω m (ohm meters) (lead) electrical conductivity | median | 0.01 S/m (siemens per meter) | highest | 4.8×10^6 S/m (siemens per meter) (lead) | lowest | 1×10^-15 S/m (siemens per meter) (sulfur) | distribution | volume magnetic susceptibility | median | -1.46×10^-5 | highest | -2.31×10^-8 (chlorine) | lowest | -2.14×10^-5 (boron) | distribution | mass magnetic susceptibility | median | -6.7×10^-9 m^3/kg (cubic meters per kilogram) | highest | -1.5×10^-9 m^3/kg (cubic meters per kilogram) (lead) | lowest | -8.7×10^-9 m^3/kg (cubic meters per kilogram) (boron) | distribution | molar magnetic susceptibility | median | -2.55×10^-10 m^3/mol (cubic meters per mole) | highest | -9.41×10^-11 m^3/mol (cubic meters per mole) (boron) | lowest | -5.11×10^-10 m^3/mol (cubic meters per mole) (chlorine) | distribution | work function | all | 4.25 eV | 4.45 eV
Reactivity
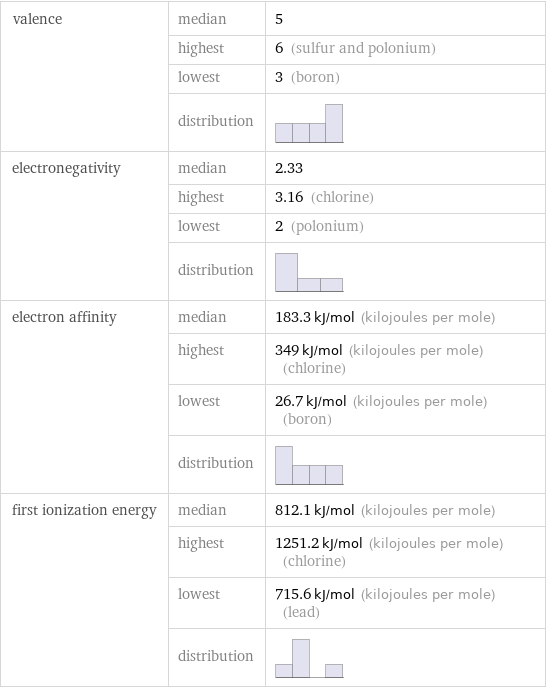
valence | median | 5 | highest | 6 (sulfur and polonium) | lowest | 3 (boron) | distribution | electronegativity | median | 2.33 | highest | 3.16 (chlorine) | lowest | 2 (polonium) | distribution | electron affinity | median | 183.3 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 26.7 kJ/mol (kilojoules per mole) (boron) | distribution | first ionization energy | median | 812.1 kJ/mol (kilojoules per mole) | highest | 1251.2 kJ/mol (kilojoules per mole) (chlorine) | lowest | 715.6 kJ/mol (kilojoules per mole) (lead) | distribution |
Atomic properties
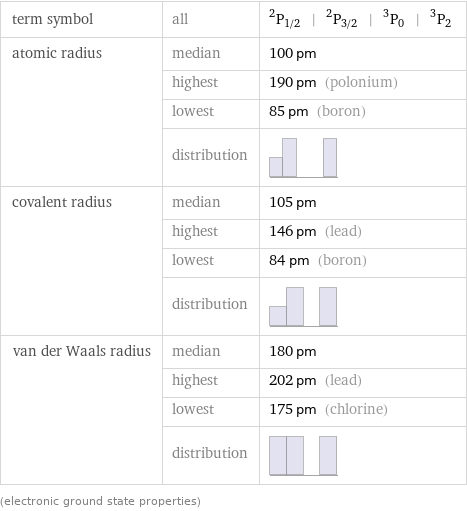
term symbol | all | ^2P_(1/2) | ^2P_(3/2) | ^3P_0 | ^3P_2 atomic radius | median | 100 pm | highest | 190 pm (polonium) | lowest | 85 pm (boron) | distribution | covalent radius | median | 105 pm | highest | 146 pm (lead) | lowest | 84 pm (boron) | distribution | van der Waals radius | median | 180 pm | highest | 202 pm (lead) | lowest | 175 pm (chlorine) | distribution | (electronic ground state properties)
Abundances
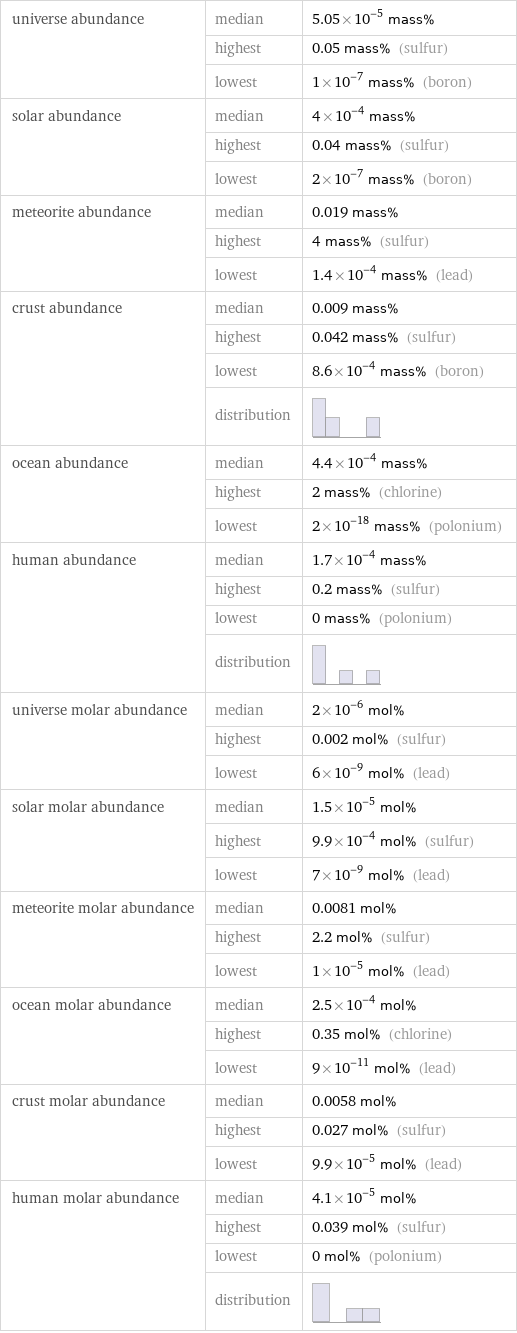
universe abundance | median | 5.05×10^-5 mass% | highest | 0.05 mass% (sulfur) | lowest | 1×10^-7 mass% (boron) solar abundance | median | 4×10^-4 mass% | highest | 0.04 mass% (sulfur) | lowest | 2×10^-7 mass% (boron) meteorite abundance | median | 0.019 mass% | highest | 4 mass% (sulfur) | lowest | 1.4×10^-4 mass% (lead) crust abundance | median | 0.009 mass% | highest | 0.042 mass% (sulfur) | lowest | 8.6×10^-4 mass% (boron) | distribution | ocean abundance | median | 4.4×10^-4 mass% | highest | 2 mass% (chlorine) | lowest | 2×10^-18 mass% (polonium) human abundance | median | 1.7×10^-4 mass% | highest | 0.2 mass% (sulfur) | lowest | 0 mass% (polonium) | distribution | universe molar abundance | median | 2×10^-6 mol% | highest | 0.002 mol% (sulfur) | lowest | 6×10^-9 mol% (lead) solar molar abundance | median | 1.5×10^-5 mol% | highest | 9.9×10^-4 mol% (sulfur) | lowest | 7×10^-9 mol% (lead) meteorite molar abundance | median | 0.0081 mol% | highest | 2.2 mol% (sulfur) | lowest | 1×10^-5 mol% (lead) ocean molar abundance | median | 2.5×10^-4 mol% | highest | 0.35 mol% (chlorine) | lowest | 9×10^-11 mol% (lead) crust molar abundance | median | 0.0058 mol% | highest | 0.027 mol% (sulfur) | lowest | 9.9×10^-5 mol% (lead) human molar abundance | median | 4.1×10^-5 mol% | highest | 0.039 mol% (sulfur) | lowest | 0 mol% (polonium) | distribution |