Input interpretation

transition metals | covalent radius
Summary
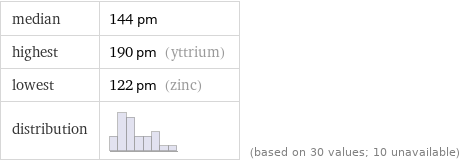
median | 144 pm highest | 190 pm (yttrium) lowest | 122 pm (zinc) distribution | | (based on 30 values; 10 unavailable)
Entities with missing values
lawrencium | rutherfordium | dubnium | seaborgium | bohrium | hassium | meitnerium | darmstadtium | roentgenium | copernicium (total: 10)
Plot
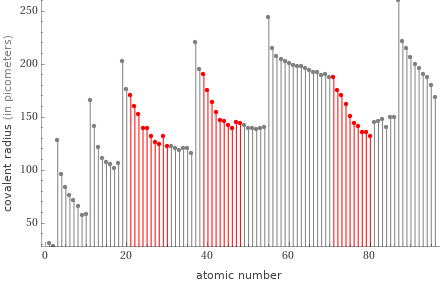
Plot
Periodic table location
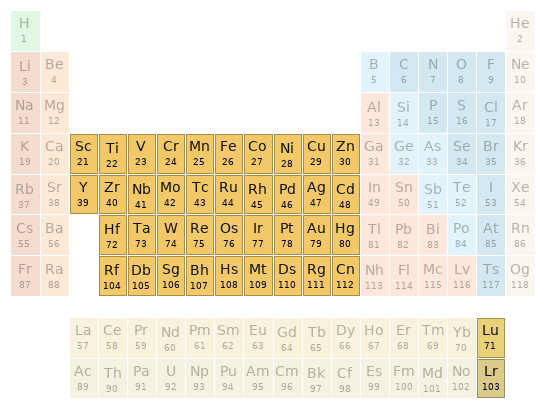
Periodic table location
Atomic radii properties
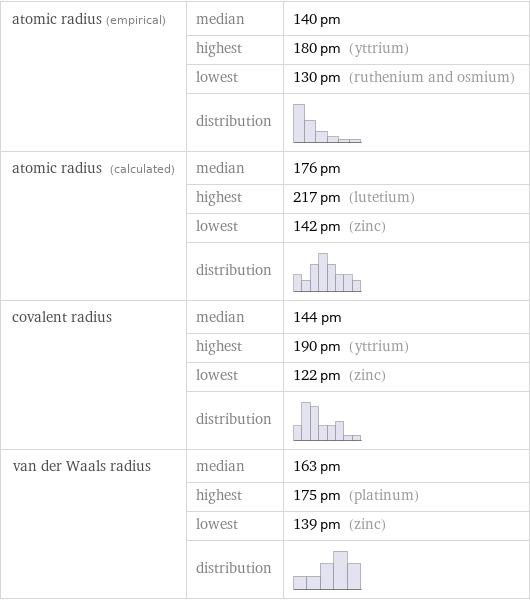
atomic radius (empirical) | median | 140 pm | highest | 180 pm (yttrium) | lowest | 130 pm (ruthenium and osmium) | distribution | atomic radius (calculated) | median | 176 pm | highest | 217 pm (lutetium) | lowest | 142 pm (zinc) | distribution | covalent radius | median | 144 pm | highest | 190 pm (yttrium) | lowest | 122 pm (zinc) | distribution | van der Waals radius | median | 163 pm | highest | 175 pm (platinum) | lowest | 139 pm (zinc) | distribution |
Units

Distribution plots
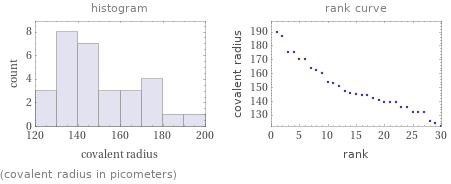
(covalent radius in picometers)
Covalent radius rankings
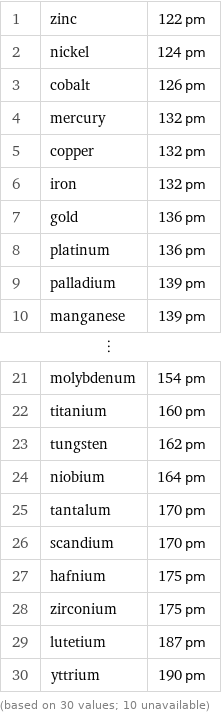
1 | zinc | 122 pm 2 | nickel | 124 pm 3 | cobalt | 126 pm 4 | mercury | 132 pm 5 | copper | 132 pm 6 | iron | 132 pm 7 | gold | 136 pm 8 | platinum | 136 pm 9 | palladium | 139 pm 10 | manganese | 139 pm ⋮ | | 21 | molybdenum | 154 pm 22 | titanium | 160 pm 23 | tungsten | 162 pm 24 | niobium | 164 pm 25 | tantalum | 170 pm 26 | scandium | 170 pm 27 | hafnium | 175 pm 28 | zirconium | 175 pm 29 | lutetium | 187 pm 30 | yttrium | 190 pm (based on 30 values; 10 unavailable)
Unit conversions for median covalent radius 144 pm
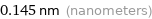
0.145 nm (nanometers)
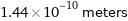
1.44×10^-10 meters
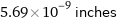
5.69×10^-9 inches
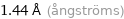
1.44 Å (ångströms)
Comparisons for median covalent radius 144 pm
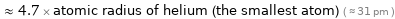
≈ 4.7 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
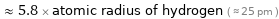
≈ 5.8 × atomic radius of hydrogen ( ≈ 25 pm )