Input interpretation
hydrogen peroxide
Chemical names and formulas

formula | H_2O_2 name | hydrogen peroxide alternate names | albone | hydrogen dioxide | hydrogen peroxide (2:2) | hydroperoxide mass fractions | H (hydrogen) 5.93% | O (oxygen) 94.1%
Lewis structure
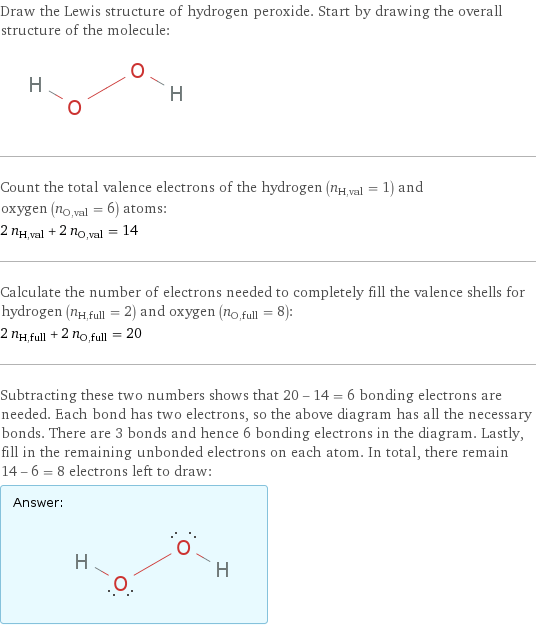
Draw the Lewis structure of hydrogen peroxide. Start by drawing the overall structure of the molecule: Count the total valence electrons of the hydrogen (n_H, val = 1) and oxygen (n_O, val = 6) atoms: 2 n_H, val + 2 n_O, val = 14 Calculate the number of electrons needed to completely fill the valence shells for hydrogen (n_H, full = 2) and oxygen (n_O, full = 8): 2 n_H, full + 2 n_O, full = 20 Subtracting these two numbers shows that 20 - 14 = 6 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 3 bonds and hence 6 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 14 - 6 = 8 electrons left to draw: Answer: | |
3D structure

3D structure
Basic properties
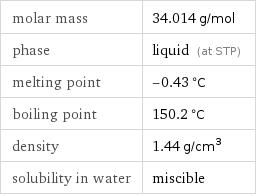
molar mass | 34.014 g/mol phase | liquid (at STP) melting point | -0.43 °C boiling point | 150.2 °C density | 1.44 g/cm^3 solubility in water | miscible
Units

Liquid properties (at STP)
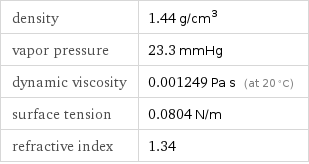
density | 1.44 g/cm^3 vapor pressure | 23.3 mmHg dynamic viscosity | 0.001249 Pa s (at 20 °C) surface tension | 0.0804 N/m refractive index | 1.34
Units

Thermodynamic properties
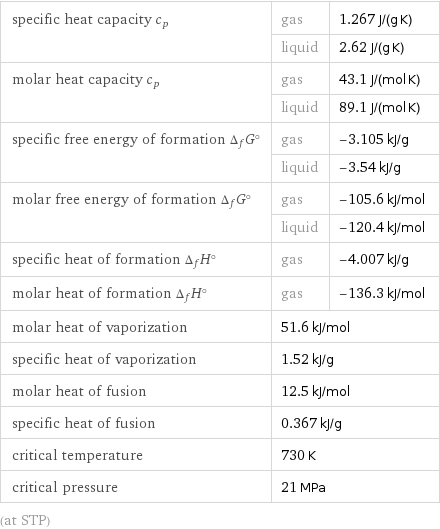
specific heat capacity c_p | gas | 1.267 J/(g K) | liquid | 2.62 J/(g K) molar heat capacity c_p | gas | 43.1 J/(mol K) | liquid | 89.1 J/(mol K) specific free energy of formation Δ_fG° | gas | -3.105 kJ/g | liquid | -3.54 kJ/g molar free energy of formation Δ_fG° | gas | -105.6 kJ/mol | liquid | -120.4 kJ/mol specific heat of formation Δ_fH° | gas | -4.007 kJ/g molar heat of formation Δ_fH° | gas | -136.3 kJ/mol molar heat of vaporization | 51.6 kJ/mol | specific heat of vaporization | 1.52 kJ/g | molar heat of fusion | 12.5 kJ/mol | specific heat of fusion | 0.367 kJ/g | critical temperature | 730 K | critical pressure | 21 MPa | (at STP)
Chemical identifiers
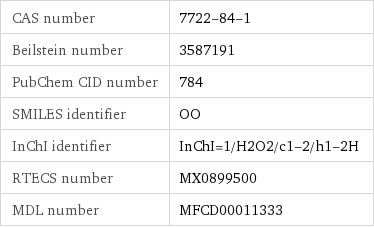
CAS number | 7722-84-1 Beilstein number | 3587191 PubChem CID number | 784 SMILES identifier | OO InChI identifier | InChI=1/H2O2/c1-2/h1-2H RTECS number | MX0899500 MDL number | MFCD00011333
NFPA label

NFPA label
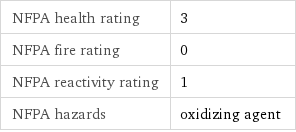
NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 1 NFPA hazards | oxidizing agent
Toxicity properties

lethal dosage | 1518 mg/kg (oral dose for rats) short-term exposure limit | 3 mg/m^3 threshold limit value | 1 ppmv

probable lethal dose for man | 600 mL (milliliters) long-term exposure limit | 1.5 mg/m^3 (over 8 hours) RTECS classes | tumorigen | drug | mutagen