Input interpretation

lawrencium(III) cation | ion class
Result
actinide ions | cations | d block ions | f block ions | group 3 ions | transition metal ions (total: 6)
Ionic radii
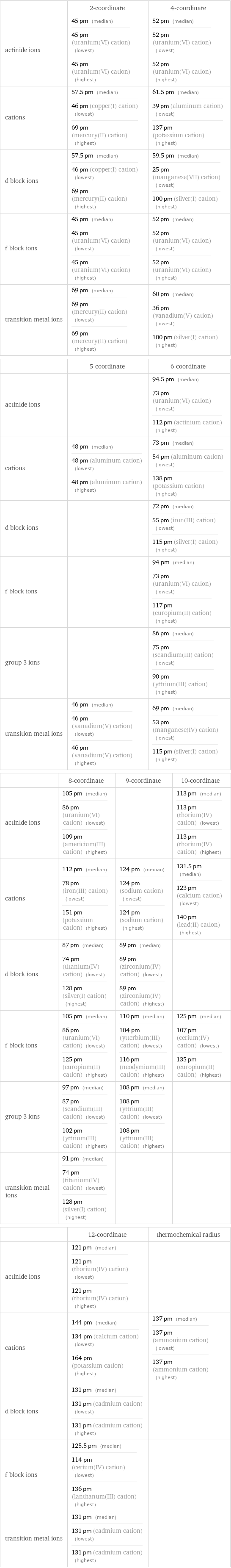
| 2-coordinate | 4-coordinate actinide ions | 45 pm (median) 45 pm (uranium(VI) cation) (lowest) 45 pm (uranium(VI) cation) (highest) | 52 pm (median) 52 pm (uranium(VI) cation) (lowest) 52 pm (uranium(VI) cation) (highest) cations | 57.5 pm (median) 46 pm (copper(I) cation) (lowest) 69 pm (mercury(II) cation) (highest) | 61.5 pm (median) 39 pm (aluminum cation) (lowest) 137 pm (potassium cation) (highest) d block ions | 57.5 pm (median) 46 pm (copper(I) cation) (lowest) 69 pm (mercury(II) cation) (highest) | 59.5 pm (median) 25 pm (manganese(VII) cation) (lowest) 100 pm (silver(I) cation) (highest) f block ions | 45 pm (median) 45 pm (uranium(VI) cation) (lowest) 45 pm (uranium(VI) cation) (highest) | 52 pm (median) 52 pm (uranium(VI) cation) (lowest) 52 pm (uranium(VI) cation) (highest) transition metal ions | 69 pm (median) 69 pm (mercury(II) cation) (lowest) 69 pm (mercury(II) cation) (highest) | 60 pm (median) 36 pm (vanadium(V) cation) (lowest) 100 pm (silver(I) cation) (highest) | 5-coordinate | 6-coordinate actinide ions | | 94.5 pm (median) 73 pm (uranium(VI) cation) (lowest) 112 pm (actinium cation) (highest) cations | 48 pm (median) 48 pm (aluminum cation) (lowest) 48 pm (aluminum cation) (highest) | 73 pm (median) 54 pm (aluminum cation) (lowest) 138 pm (potassium cation) (highest) d block ions | | 72 pm (median) 55 pm (iron(III) cation) (lowest) 115 pm (silver(I) cation) (highest) f block ions | | 94 pm (median) 73 pm (uranium(VI) cation) (lowest) 117 pm (europium(II) cation) (highest) group 3 ions | | 86 pm (median) 75 pm (scandium(III) cation) (lowest) 90 pm (yttrium(III) cation) (highest) transition metal ions | 46 pm (median) 46 pm (vanadium(V) cation) (lowest) 46 pm (vanadium(V) cation) (highest) | 69 pm (median) 53 pm (manganese(IV) cation) (lowest) 115 pm (silver(I) cation) (highest) | 8-coordinate | 9-coordinate | 10-coordinate actinide ions | 105 pm (median) 86 pm (uranium(VI) cation) (lowest) 109 pm (americium(III) cation) (highest) | | 113 pm (median) 113 pm (thorium(IV) cation) (lowest) 113 pm (thorium(IV) cation) (highest) cations | 112 pm (median) 78 pm (iron(III) cation) (lowest) 151 pm (potassium cation) (highest) | 124 pm (median) 124 pm (sodium cation) (lowest) 124 pm (sodium cation) (highest) | 131.5 pm (median) 123 pm (calcium cation) (lowest) 140 pm (lead(II) cation) (highest) d block ions | 87 pm (median) 74 pm (titanium(IV) cation) (lowest) 128 pm (silver(I) cation) (highest) | 89 pm (median) 89 pm (zirconium(IV) cation) (lowest) 89 pm (zirconium(IV) cation) (highest) | f block ions | 105 pm (median) 86 pm (uranium(VI) cation) (lowest) 125 pm (europium(II) cation) (highest) | 110 pm (median) 104 pm (ytterbium(III) cation) (lowest) 116 pm (neodymium(III) cation) (highest) | 125 pm (median) 107 pm (cerium(IV) cation) (lowest) 135 pm (europium(II) cation) (highest) group 3 ions | 97 pm (median) 87 pm (scandium(III) cation) (lowest) 102 pm (yttrium(III) cation) (highest) | 108 pm (median) 108 pm (yttrium(III) cation) (lowest) 108 pm (yttrium(III) cation) (highest) | transition metal ions | 91 pm (median) 74 pm (titanium(IV) cation) (lowest) 128 pm (silver(I) cation) (highest) | | | 12-coordinate | thermochemical radius actinide ions | 121 pm (median) 121 pm (thorium(IV) cation) (lowest) 121 pm (thorium(IV) cation) (highest) | cations | 144 pm (median) 134 pm (calcium cation) (lowest) 164 pm (potassium cation) (highest) | 137 pm (median) 137 pm (ammonium cation) (lowest) 137 pm (ammonium cation) (highest) d block ions | 131 pm (median) 131 pm (cadmium cation) (lowest) 131 pm (cadmium cation) (highest) | f block ions | 125.5 pm (median) 114 pm (cerium(IV) cation) (lowest) 136 pm (lanthanum(III) cation) (highest) | transition metal ions | 131 pm (median) 131 pm (cadmium cation) (lowest) 131 pm (cadmium cation) (highest) |