Input interpretation

carbon allotropes | relative molecular mass
Summary
![median | 12.011 highest | 840.77 ([5, 6]-fullerene-C70) lowest | 12.011 (3 chemicals) distribution |](../image_source/caba25342e54704d8882f1446a052e4b.png)
median | 12.011 highest | 840.77 ([5, 6]-fullerene-C70) lowest | 12.011 (3 chemicals) distribution |
Ranked values
![| | visual | ratios | 5 | activated charcoal | | 0.014286 | 1 4 | diamond | | 0.014286 | 1 3 | graphite | | 0.014286 | 1 2 | buckminsterfullerene | | 0.8571 | 60 1 | [5, 6]-fullerene-C70 | | 1 | 70](../image_source/d5cb13974a24e0ba36a57188a60f0a39.png)
| | visual | ratios | 5 | activated charcoal | | 0.014286 | 1 4 | diamond | | 0.014286 | 1 3 | graphite | | 0.014286 | 1 2 | buckminsterfullerene | | 0.8571 | 60 1 | [5, 6]-fullerene-C70 | | 1 | 70
Relative molecular mass rankings
![1 | graphite | 12.011 2 | diamond | 12.011 3 | activated charcoal | 12.011 4 | buckminsterfullerene | 720.66 5 | [5, 6]-fullerene-C70 | 840.77](../image_source/f0777fceeed4a3ba0d90902025f03bab.png)
1 | graphite | 12.011 2 | diamond | 12.011 3 | activated charcoal | 12.011 4 | buckminsterfullerene | 720.66 5 | [5, 6]-fullerene-C70 | 840.77
Comparisons for median relative molecular mass 12.011

≈ ( 0.062 ≈ 1/16 ) × relative molecular mass of caffeine ( ≈ 194 )
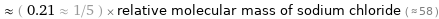
≈ ( 0.21 ≈ 1/5 ) × relative molecular mass of sodium chloride ( ≈ 58 )
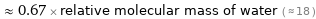
≈ 0.67 × relative molecular mass of water ( ≈ 18 )
Corresponding quantities
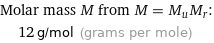
Molar mass M from M = M_uM_r: | 12 g/mol (grams per mole)

Molecular mass m from m = M_rM_u/N_A: | 2×10^-23 grams | 2×10^-26 kg (kilograms)