Input interpretation

group 13 elements | phase at STP
Summary
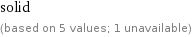
solid (based on 5 values; 1 unavailable)
Entity with missing value
nihonium
Table
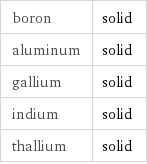
boron | solid aluminum | solid gallium | solid indium | solid thallium | solid
Thermodynamic properties
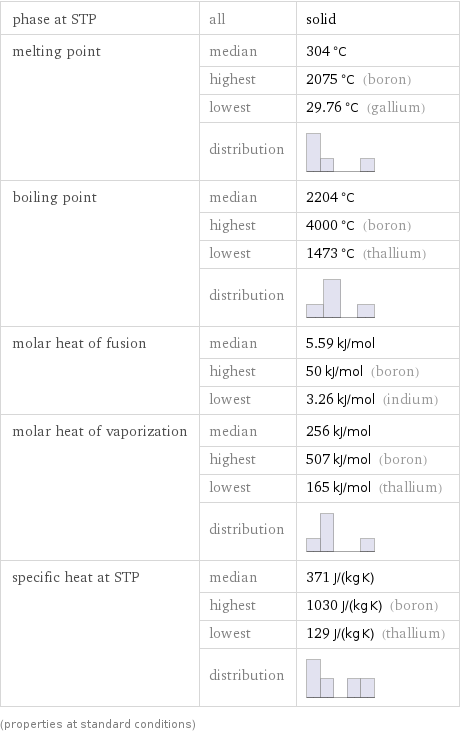
phase at STP | all | solid melting point | median | 304 °C | highest | 2075 °C (boron) | lowest | 29.76 °C (gallium) | distribution | boiling point | median | 2204 °C | highest | 4000 °C (boron) | lowest | 1473 °C (thallium) | distribution | molar heat of fusion | median | 5.59 kJ/mol | highest | 50 kJ/mol (boron) | lowest | 3.26 kJ/mol (indium) molar heat of vaporization | median | 256 kJ/mol | highest | 507 kJ/mol (boron) | lowest | 165 kJ/mol (thallium) | distribution | specific heat at STP | median | 371 J/(kg K) | highest | 1030 J/(kg K) (boron) | lowest | 129 J/(kg K) (thallium) | distribution | (properties at standard conditions)