Input interpretation
sodium bromate | copper
Chemical names and formulas
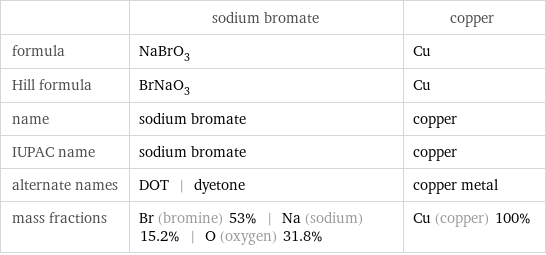
| sodium bromate | copper formula | NaBrO_3 | Cu Hill formula | BrNaO_3 | Cu name | sodium bromate | copper IUPAC name | sodium bromate | copper alternate names | DOT | dyetone | copper metal mass fractions | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8% | Cu (copper) 100%
Structure diagrams
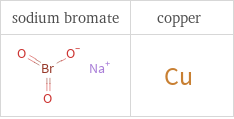
Structure diagrams
Basic properties
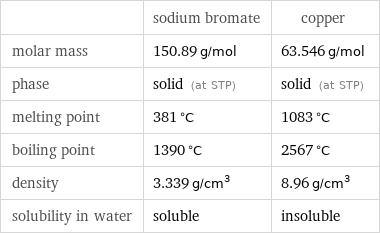
| sodium bromate | copper molar mass | 150.89 g/mol | 63.546 g/mol phase | solid (at STP) | solid (at STP) melting point | 381 °C | 1083 °C boiling point | 1390 °C | 2567 °C density | 3.339 g/cm^3 | 8.96 g/cm^3 solubility in water | soluble | insoluble
Units

Thermodynamic properties
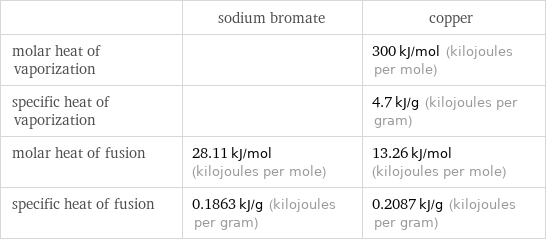
| sodium bromate | copper molar heat of vaporization | | 300 kJ/mol (kilojoules per mole) specific heat of vaporization | | 4.7 kJ/g (kilojoules per gram) molar heat of fusion | 28.11 kJ/mol (kilojoules per mole) | 13.26 kJ/mol (kilojoules per mole) specific heat of fusion | 0.1863 kJ/g (kilojoules per gram) | 0.2087 kJ/g (kilojoules per gram)
Solid properties (at STP)

| sodium bromate | copper density | 3.339 g/cm^3 | 8.96 g/cm^3 vapor pressure | | 1 mmHg
Units

Chemical identifiers
![| sodium bromate | copper CAS number | 7789-38-0 | 7440-50-8 PubChem CID number | 23668195 | 23978 PubChem SID number | 24853461 | 24852148 SMILES identifier | [O-]Br(=O)=O.[Na+] | [Cu]](../image_source/f2ca7f67e3db17c0c70b02ba42391a11.png)
| sodium bromate | copper CAS number | 7789-38-0 | 7440-50-8 PubChem CID number | 23668195 | 23978 PubChem SID number | 24853461 | 24852148 SMILES identifier | [O-]Br(=O)=O.[Na+] | [Cu]
NFPA label

NFPA label