Input interpretation
protactinium (chemical element)
Periodic table location
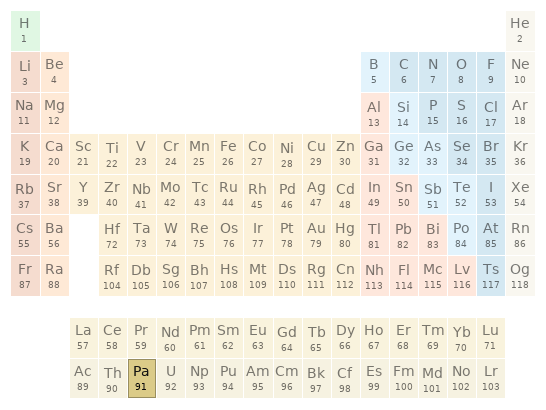
Periodic table location
Image
Image
Basic elemental properties
![atomic symbol | Pa atomic number | 91 short electronic configuration | [Rn]7s^25f^26d^1 Aufbau diagram | 6d 5f 7s block | f period | 7 atomic mass | 231.03588 u (unified atomic mass units) (atomic mass number given for longest-lived isotope) half-life | 32790 years](../image_source/fe01154d008774de9bd99238382b6184.png)
atomic symbol | Pa atomic number | 91 short electronic configuration | [Rn]7s^25f^26d^1 Aufbau diagram | 6d 5f 7s block | f period | 7 atomic mass | 231.03588 u (unified atomic mass units) (atomic mass number given for longest-lived isotope) half-life | 32790 years
Thermodynamic properties
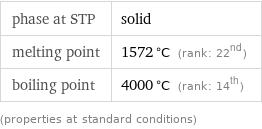
phase at STP | solid melting point | 1572 °C (rank: 22nd) boiling point | 4000 °C (rank: 14th) (properties at standard conditions)
Material properties
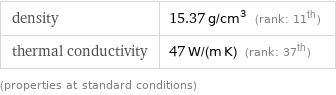
density | 15.37 g/cm^3 (rank: 11th) thermal conductivity | 47 W/(m K) (rank: 37th) (properties at standard conditions)
Units

Electromagnetic properties
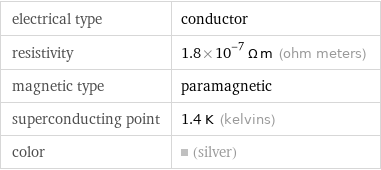
electrical type | conductor resistivity | 1.8×10^-7 Ω m (ohm meters) magnetic type | paramagnetic superconducting point | 1.4 K (kelvins) color | (silver)
Reactivity
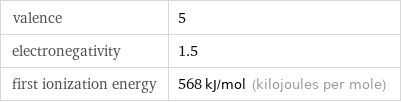
valence | 5 electronegativity | 1.5 first ionization energy | 568 kJ/mol (kilojoules per mole)
Atomic properties

term symbol | ^4K_(11/2) atomic radius | 180 pm (electronic ground state properties)
Units

Abundances
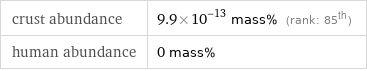
crust abundance | 9.9×10^-13 mass% (rank: 85th) human abundance | 0 mass%
Units

Nuclear properties
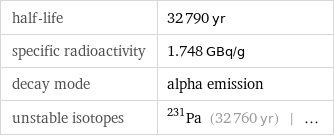
half-life | 32790 yr specific radioactivity | 1.748 GBq/g decay mode | alpha emission unstable isotopes | Pa-231 (32760 yr) | ...
Units

Identifiers

CAS number | 7440-13-3