Input interpretation
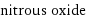
nitrous oxide
Chemical names and formulas

formula | N_2O name | nitrous oxide alternate names | dinitrogen monoxide | dinitrogen oxide | factitious air | Freon 744a | laughing gas | R-744a mass fractions | N (nitrogen) 63.6% | O (oxygen) 36.4%
Lewis structure

Draw the Lewis structure of nitrous oxide. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the nitrogen (n_N, val = 5) and oxygen (n_O, val = 6) atoms: 2 n_N, val + n_O, val = 16 Calculate the number of electrons needed to completely fill the valence shells for nitrogen (n_N, full = 8) and oxygen (n_O, full = 8): 2 n_N, full + n_O, full = 24 Subtracting these two numbers shows that 24 - 16 = 8 bonding electrons are needed. Each bond has two electrons, so in addition to the 2 bonds already present in the diagram add 2 bonds. To minimize formal charge oxygen wants 2 bonds and nitrogen wants 3 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: To fully fill its valence shell, nitrogen will donate one of its electrons, allowing it to form four bonds (the maximum number an element on period 2 can form). Fill in the 2 bonds by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms: Answer: | |
3D structure

3D structure
Basic properties
molar mass | 44.013 g/mol phase | gas (at STP) melting point | -91 °C boiling point | -88 °C density | 0.001799 g/cm^3 (at 25 °C) dielectric constant | 1.001
Gas properties (at STP)
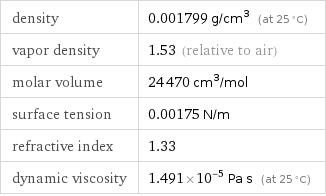
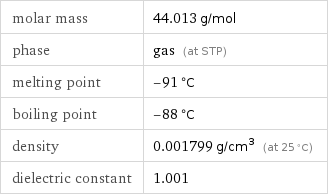
density | 0.001799 g/cm^3 (at 25 °C) vapor density | 1.53 (relative to air) molar volume | 24470 cm^3/mol surface tension | 0.00175 N/m refractive index | 1.33 dynamic viscosity | 1.491×10^-5 Pa s (at 25 °C)
Units

Thermodynamic properties
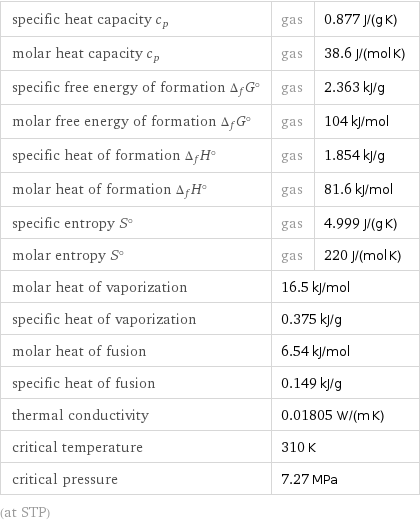
specific heat capacity c_p | gas | 0.877 J/(g K) molar heat capacity c_p | gas | 38.6 J/(mol K) specific free energy of formation Δ_fG° | gas | 2.363 kJ/g molar free energy of formation Δ_fG° | gas | 104 kJ/mol specific heat of formation Δ_fH° | gas | 1.854 kJ/g molar heat of formation Δ_fH° | gas | 81.6 kJ/mol specific entropy S° | gas | 4.999 J/(g K) molar entropy S° | gas | 220 J/(mol K) molar heat of vaporization | 16.5 kJ/mol | specific heat of vaporization | 0.375 kJ/g | molar heat of fusion | 6.54 kJ/mol | specific heat of fusion | 0.149 kJ/g | thermal conductivity | 0.01805 W/(m K) | critical temperature | 310 K | critical pressure | 7.27 MPa | (at STP)
Phase diagram
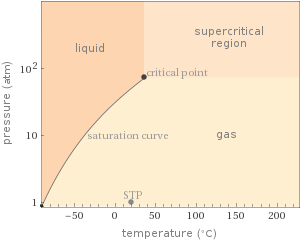
Phase diagram
Units
