Input interpretation
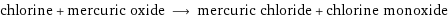
chlorine + mercuric oxide ⟶ mercuric chloride + chlorine monoxide
Balanced equation
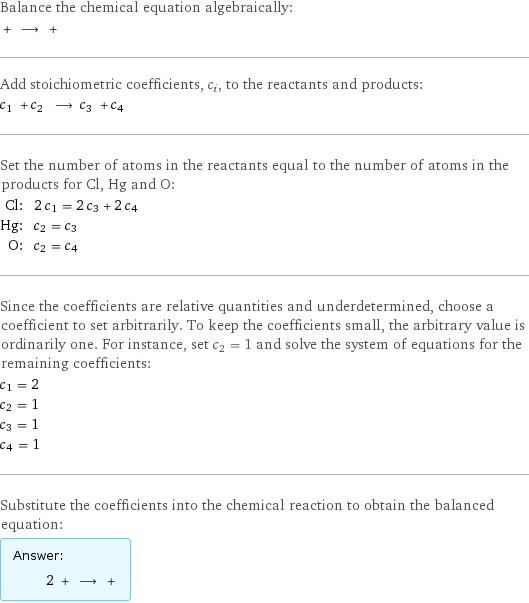
Balance the chemical equation algebraically: + ⟶ + Add stoichiometric coefficients, c_i, to the reactants and products: c_1 + c_2 ⟶ c_3 + c_4 Set the number of atoms in the reactants equal to the number of atoms in the products for Cl, Hg and O: Cl: | 2 c_1 = 2 c_3 + 2 c_4 Hg: | c_2 = c_3 O: | c_2 = c_4 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_2 = 1 and solve the system of equations for the remaining coefficients: c_1 = 2 c_2 = 1 c_3 = 1 c_4 = 1 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | 2 + ⟶ +
Structures
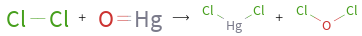
+ ⟶ +
Names
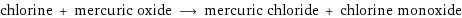
chlorine + mercuric oxide ⟶ mercuric chloride + chlorine monoxide
Reaction thermodynamics
Enthalpy
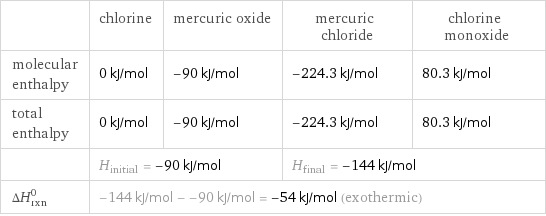
| chlorine | mercuric oxide | mercuric chloride | chlorine monoxide molecular enthalpy | 0 kJ/mol | -90 kJ/mol | -224.3 kJ/mol | 80.3 kJ/mol total enthalpy | 0 kJ/mol | -90 kJ/mol | -224.3 kJ/mol | 80.3 kJ/mol | H_initial = -90 kJ/mol | | H_final = -144 kJ/mol | ΔH_rxn^0 | -144 kJ/mol - -90 kJ/mol = -54 kJ/mol (exothermic) | | |
Gibbs free energy
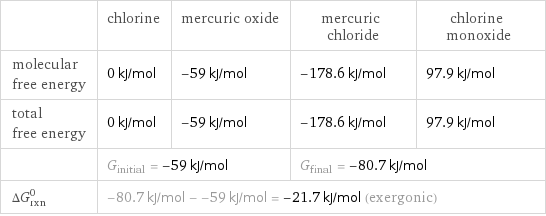
| chlorine | mercuric oxide | mercuric chloride | chlorine monoxide molecular free energy | 0 kJ/mol | -59 kJ/mol | -178.6 kJ/mol | 97.9 kJ/mol total free energy | 0 kJ/mol | -59 kJ/mol | -178.6 kJ/mol | 97.9 kJ/mol | G_initial = -59 kJ/mol | | G_final = -80.7 kJ/mol | ΔG_rxn^0 | -80.7 kJ/mol - -59 kJ/mol = -21.7 kJ/mol (exergonic) | | |
Chemical names and formulas
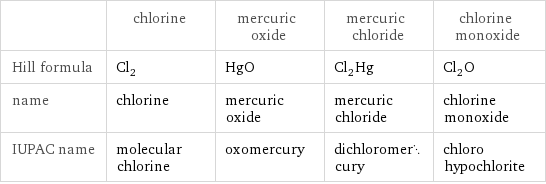
| chlorine | mercuric oxide | mercuric chloride | chlorine monoxide Hill formula | Cl_2 | HgO | Cl_2Hg | Cl_2O name | chlorine | mercuric oxide | mercuric chloride | chlorine monoxide IUPAC name | molecular chlorine | oxomercury | dichloromercury | chloro hypochlorite
Substance properties
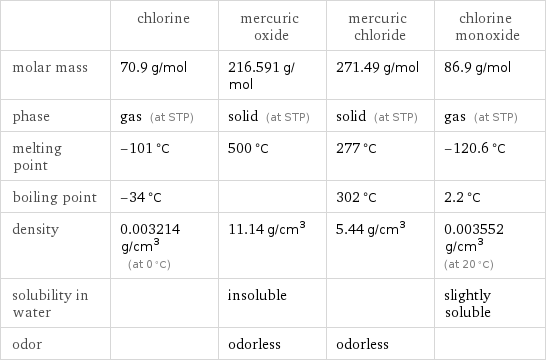
| chlorine | mercuric oxide | mercuric chloride | chlorine monoxide molar mass | 70.9 g/mol | 216.591 g/mol | 271.49 g/mol | 86.9 g/mol phase | gas (at STP) | solid (at STP) | solid (at STP) | gas (at STP) melting point | -101 °C | 500 °C | 277 °C | -120.6 °C boiling point | -34 °C | | 302 °C | 2.2 °C density | 0.003214 g/cm^3 (at 0 °C) | 11.14 g/cm^3 | 5.44 g/cm^3 | 0.003552 g/cm^3 (at 20 °C) solubility in water | | insoluble | | slightly soluble odor | | odorless | odorless |
Units
