Input interpretation
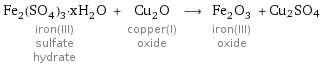
Fe_2(SO_4)_3·xH_2O iron(III) sulfate hydrate + Cu_2O copper(I) oxide ⟶ Fe_2O_3 iron(III) oxide + Cu2SO4
Chemical names and formulas
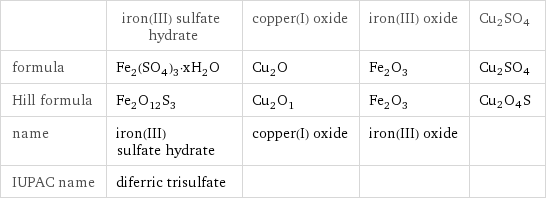
| iron(III) sulfate hydrate | copper(I) oxide | iron(III) oxide | Cu2SO4 formula | Fe_2(SO_4)_3·xH_2O | Cu_2O | Fe_2O_3 | Cu2SO4 Hill formula | Fe_2O_12S_3 | Cu_2O_1 | Fe_2O_3 | Cu2O4S name | iron(III) sulfate hydrate | copper(I) oxide | iron(III) oxide | IUPAC name | diferric trisulfate | | |
Substance properties
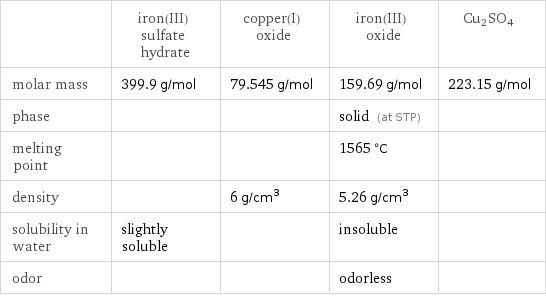
| iron(III) sulfate hydrate | copper(I) oxide | iron(III) oxide | Cu2SO4 molar mass | 399.9 g/mol | 79.545 g/mol | 159.69 g/mol | 223.15 g/mol phase | | | solid (at STP) | melting point | | | 1565 °C | density | | 6 g/cm^3 | 5.26 g/cm^3 | solubility in water | slightly soluble | | insoluble | odor | | | odorless |
Units
