Input interpretation
deuterium iodide | 1-butene
Chemical names and formulas
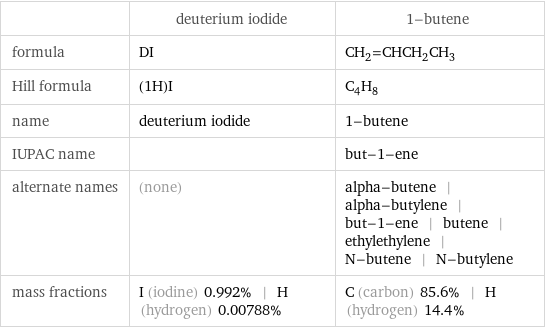
| deuterium iodide | 1-butene formula | DI | CH_2=CHCH_2CH_3 Hill formula | (1H)I | C_4H_8 name | deuterium iodide | 1-butene IUPAC name | | but-1-ene alternate names | (none) | alpha-butene | alpha-butylene | but-1-ene | butene | ethylethylene | N-butene | N-butylene mass fractions | I (iodine) 0.992% | H (hydrogen) 0.00788% | C (carbon) 85.6% | H (hydrogen) 14.4%
Structure diagrams
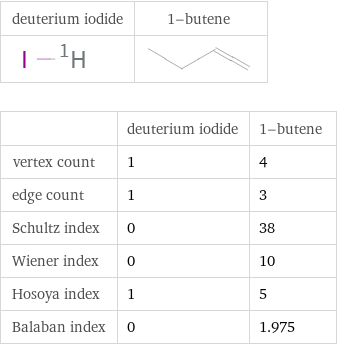
| deuterium iodide | 1-butene vertex count | 1 | 4 edge count | 1 | 3 Schultz index | 0 | 38 Wiener index | 0 | 10 Hosoya index | 1 | 5 Balaban index | 0 | 1.975
3D structure
3D structure
Basic properties
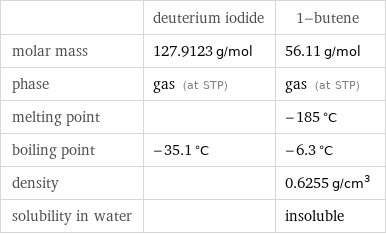
| deuterium iodide | 1-butene molar mass | 127.9123 g/mol | 56.11 g/mol phase | gas (at STP) | gas (at STP) melting point | | -185 °C boiling point | -35.1 °C | -6.3 °C density | | 0.6255 g/cm^3 solubility in water | | insoluble
Units

Gas properties (at STP)
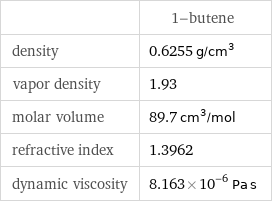
| 1-butene density | 0.6255 g/cm^3 vapor density | 1.93 molar volume | 89.7 cm^3/mol refractive index | 1.3962 dynamic viscosity | 8.163×10^-6 Pa s
Units
Thermodynamic properties
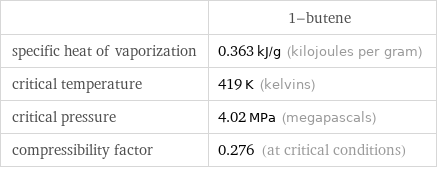
| 1-butene specific heat of vaporization | 0.363 kJ/g (kilojoules per gram) critical temperature | 419 K (kelvins) critical pressure | 4.02 MPa (megapascals) compressibility factor | 0.276 (at critical conditions)