Input interpretation
zinc cation | mercury(II) cation | gold(III) cation
Structure diagrams

Structure diagrams
General properties

| zinc cation | mercury(II) cation | gold(III) cation formula | Zn^(2+) | Hg^(2+) | Au^(3+) net ionic charge | +2 | +2 | +3 alternate names | zinc(II) | zinc(II) cation | zinc(2+) | mercuric | mercury(II) | mercury(2+) | gold(III) | gold(3+)
Ionic radii
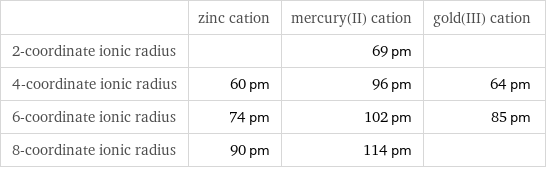
| zinc cation | mercury(II) cation | gold(III) cation 2-coordinate ionic radius | | 69 pm | 4-coordinate ionic radius | 60 pm | 96 pm | 64 pm 6-coordinate ionic radius | 74 pm | 102 pm | 85 pm 8-coordinate ionic radius | 90 pm | 114 pm |
Units

Other properties
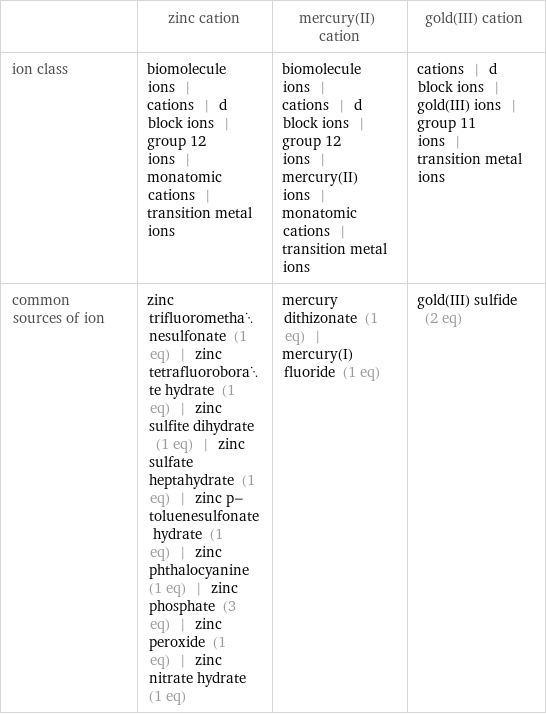
| zinc cation | mercury(II) cation | gold(III) cation ion class | biomolecule ions | cations | d block ions | group 12 ions | monatomic cations | transition metal ions | biomolecule ions | cations | d block ions | group 12 ions | mercury(II) ions | monatomic cations | transition metal ions | cations | d block ions | gold(III) ions | group 11 ions | transition metal ions common sources of ion | zinc trifluoromethanesulfonate (1 eq) | zinc tetrafluoroborate hydrate (1 eq) | zinc sulfite dihydrate (1 eq) | zinc sulfate heptahydrate (1 eq) | zinc p-toluenesulfonate hydrate (1 eq) | zinc phthalocyanine (1 eq) | zinc phosphate (3 eq) | zinc peroxide (1 eq) | zinc nitrate hydrate (1 eq) | mercury dithizonate (1 eq) | mercury(I) fluoride (1 eq) | gold(III) sulfide (2 eq)