Input interpretation

polar protic solvents | molar volume
Summary
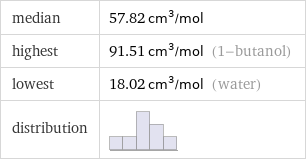
median | 57.82 cm^3/mol highest | 91.51 cm^3/mol (1-butanol) lowest | 18.02 cm^3/mol (water) distribution |
Units

Ranked values
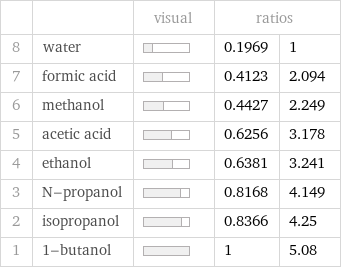
| | visual | ratios | 8 | water | | 0.1969 | 1 7 | formic acid | | 0.4123 | 2.094 6 | methanol | | 0.4427 | 2.249 5 | acetic acid | | 0.6256 | 3.178 4 | ethanol | | 0.6381 | 3.241 3 | N-propanol | | 0.8168 | 4.149 2 | isopropanol | | 0.8366 | 4.25 1 | 1-butanol | | 1 | 5.08
Molar volume rankings
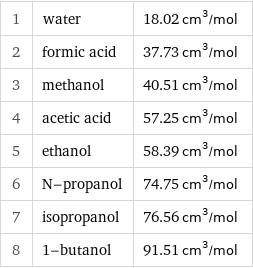
1 | water | 18.02 cm^3/mol 2 | formic acid | 37.73 cm^3/mol 3 | methanol | 40.51 cm^3/mol 4 | acetic acid | 57.25 cm^3/mol 5 | ethanol | 58.39 cm^3/mol 6 | N-propanol | 74.75 cm^3/mol 7 | isopropanol | 76.56 cm^3/mol 8 | 1-butanol | 91.51 cm^3/mol
Unit conversions for median molar volume 57.82 cm^3/mol
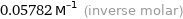
0.05782 M^(-1) (inverse molar)
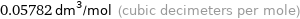
0.05782 dm^3/mol (cubic decimeters per mole)
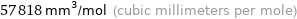
57818 mm^3/mol (cubic millimeters per mole)
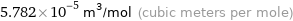
5.782×10^-5 m^3/mol (cubic meters per mole)
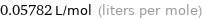
0.05782 L/mol (liters per mole)
Comparison for median molar volume 57.82 cm^3/mol
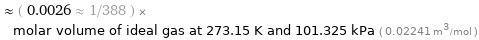
≈ ( 0.0026 ≈ 1/388 ) × molar volume of ideal gas at 273.15 K and 101.325 kPa ( 0.02241 m^3/mol )