Input interpretation
barium iodate | iron(III) oxalate
Chemical names and formulas
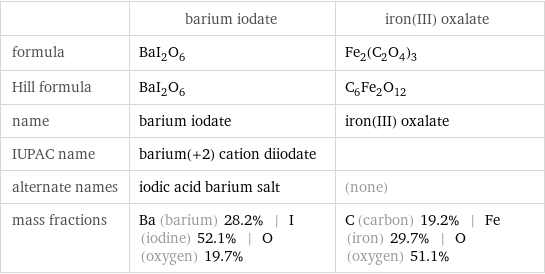
| barium iodate | iron(III) oxalate formula | BaI_2O_6 | Fe_2(C_2O_4)_3 Hill formula | BaI_2O_6 | C_6Fe_2O_12 name | barium iodate | iron(III) oxalate IUPAC name | barium(+2) cation diiodate | alternate names | iodic acid barium salt | (none) mass fractions | Ba (barium) 28.2% | I (iodine) 52.1% | O (oxygen) 19.7% | C (carbon) 19.2% | Fe (iron) 29.7% | O (oxygen) 51.1%
Structure diagrams
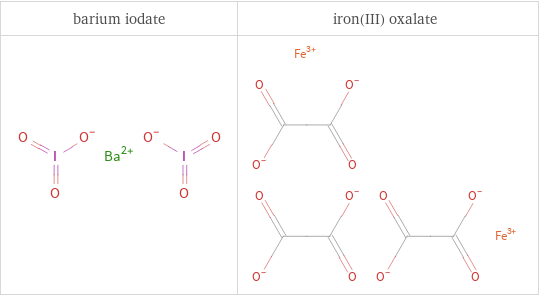
Structure diagrams
Basic properties
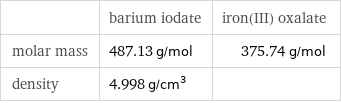
| barium iodate | iron(III) oxalate molar mass | 487.13 g/mol | 375.74 g/mol density | 4.998 g/cm^3 |
Units
