Input interpretation
rare earth metals
Members

scandium | yttrium | lanthanum | cerium | praseodymium | neodymium | promethium | samarium | europium | gadolinium | terbium | dysprosium | holmium | erbium | thulium | ytterbium | lutetium (total: 17)
Periodic table location
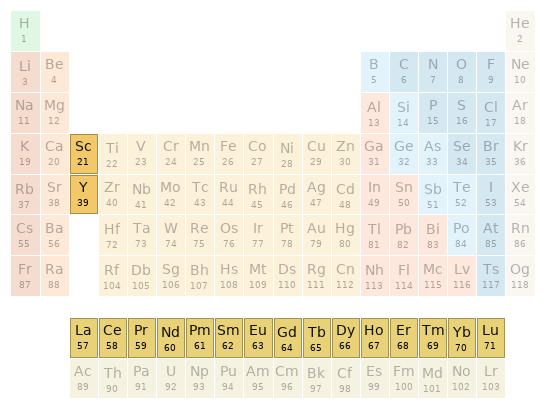
Periodic table location
Images
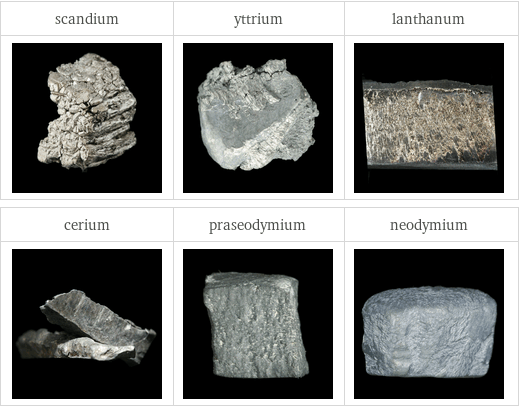
Images
Basic elemental properties
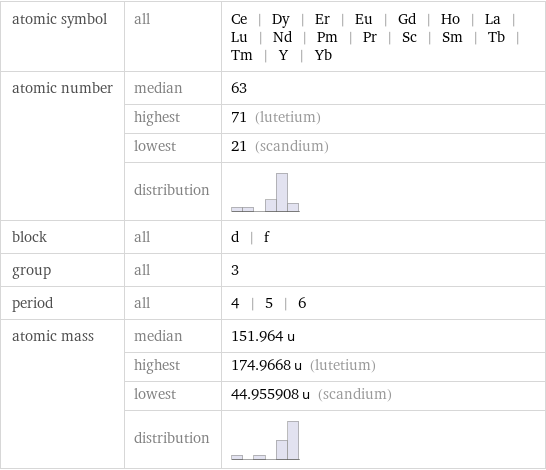
atomic symbol | all | Ce | Dy | Er | Eu | Gd | Ho | La | Lu | Nd | Pm | Pr | Sc | Sm | Tb | Tm | Y | Yb atomic number | median | 63 | highest | 71 (lutetium) | lowest | 21 (scandium) | distribution | block | all | d | f group | all | 3 period | all | 4 | 5 | 6 atomic mass | median | 151.964 u | highest | 174.9668 u (lutetium) | lowest | 44.955908 u (scandium) | distribution |
Thermodynamic properties

phase at STP | all | solid melting point | median | 1313 °C | highest | 1663 °C (lutetium) | lowest | 798 °C (cerium) | distribution | boiling point | median | 3000 °C | highest | 3464 °C (lanthanum) | lowest | 1196 °C (ytterbium) | distribution | molar heat of fusion | median | 10 kJ/mol | highest | 22 kJ/mol (lutetium) | lowest | 5.5 kJ/mol (cerium) | distribution | molar heat of vaporization | median | 290 kJ/mol | highest | 415 kJ/mol (lutetium) | lowest | 160 kJ/mol (ytterbium) | distribution | specific heat at STP | median | 186 J/(kg K) | highest | 567 J/(kg K) (scandium) | lowest | 154 J/(kg K) (ytterbium and lutetium) | distribution | Néel point | median | 90.5 K | highest | 230 K (terbium) | lowest | 12.5 K (cerium) | distribution | (properties at standard conditions)
Material properties
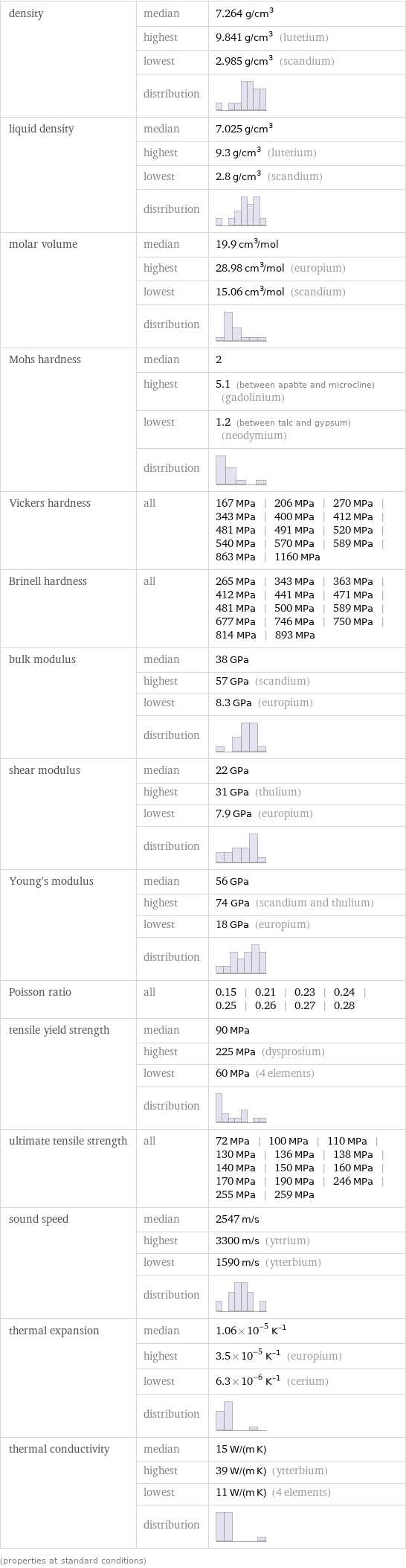
density | median | 7.264 g/cm^3 | highest | 9.841 g/cm^3 (lutetium) | lowest | 2.985 g/cm^3 (scandium) | distribution | liquid density | median | 7.025 g/cm^3 | highest | 9.3 g/cm^3 (lutetium) | lowest | 2.8 g/cm^3 (scandium) | distribution | molar volume | median | 19.9 cm^3/mol | highest | 28.98 cm^3/mol (europium) | lowest | 15.06 cm^3/mol (scandium) | distribution | Mohs hardness | median | 2 | highest | 5.1 (between apatite and microcline) (gadolinium) | lowest | 1.2 (between talc and gypsum) (neodymium) | distribution | Vickers hardness | all | 167 MPa | 206 MPa | 270 MPa | 343 MPa | 400 MPa | 412 MPa | 481 MPa | 491 MPa | 520 MPa | 540 MPa | 570 MPa | 589 MPa | 863 MPa | 1160 MPa Brinell hardness | all | 265 MPa | 343 MPa | 363 MPa | 412 MPa | 441 MPa | 471 MPa | 481 MPa | 500 MPa | 589 MPa | 677 MPa | 746 MPa | 750 MPa | 814 MPa | 893 MPa bulk modulus | median | 38 GPa | highest | 57 GPa (scandium) | lowest | 8.3 GPa (europium) | distribution | shear modulus | median | 22 GPa | highest | 31 GPa (thulium) | lowest | 7.9 GPa (europium) | distribution | Young's modulus | median | 56 GPa | highest | 74 GPa (scandium and thulium) | lowest | 18 GPa (europium) | distribution | Poisson ratio | all | 0.15 | 0.21 | 0.23 | 0.24 | 0.25 | 0.26 | 0.27 | 0.28 tensile yield strength | median | 90 MPa | highest | 225 MPa (dysprosium) | lowest | 60 MPa (4 elements) | distribution | ultimate tensile strength | all | 72 MPa | 100 MPa | 110 MPa | 130 MPa | 136 MPa | 138 MPa | 140 MPa | 150 MPa | 160 MPa | 170 MPa | 190 MPa | 246 MPa | 255 MPa | 259 MPa sound speed | median | 2547 m/s | highest | 3300 m/s (yttrium) | lowest | 1590 m/s (ytterbium) | distribution | thermal expansion | median | 1.06×10^-5 K^(-1) | highest | 3.5×10^-5 K^(-1) (europium) | lowest | 6.3×10^-6 K^(-1) (cerium) | distribution | thermal conductivity | median | 15 W/(m K) | highest | 39 W/(m K) (ytterbium) | lowest | 11 W/(m K) (4 elements) | distribution | (properties at standard conditions)
Electromagnetic properties
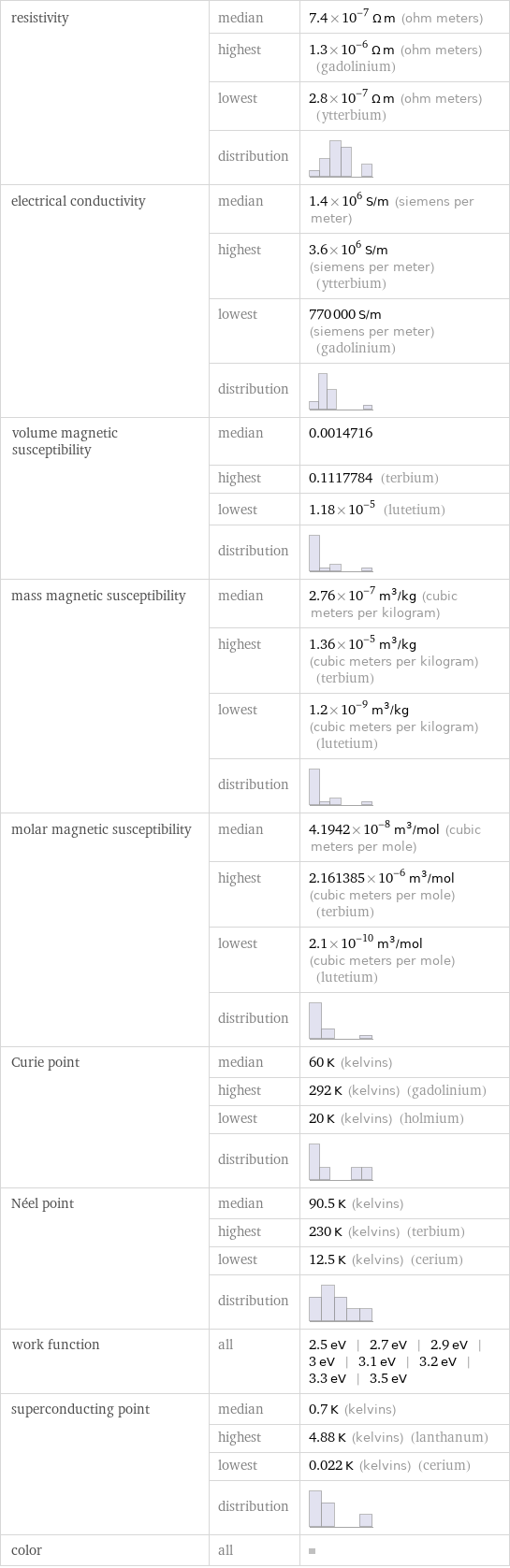
resistivity | median | 7.4×10^-7 Ω m (ohm meters) | highest | 1.3×10^-6 Ω m (ohm meters) (gadolinium) | lowest | 2.8×10^-7 Ω m (ohm meters) (ytterbium) | distribution | electrical conductivity | median | 1.4×10^6 S/m (siemens per meter) | highest | 3.6×10^6 S/m (siemens per meter) (ytterbium) | lowest | 770000 S/m (siemens per meter) (gadolinium) | distribution | volume magnetic susceptibility | median | 0.0014716 | highest | 0.1117784 (terbium) | lowest | 1.18×10^-5 (lutetium) | distribution | mass magnetic susceptibility | median | 2.76×10^-7 m^3/kg (cubic meters per kilogram) | highest | 1.36×10^-5 m^3/kg (cubic meters per kilogram) (terbium) | lowest | 1.2×10^-9 m^3/kg (cubic meters per kilogram) (lutetium) | distribution | molar magnetic susceptibility | median | 4.1942×10^-8 m^3/mol (cubic meters per mole) | highest | 2.161385×10^-6 m^3/mol (cubic meters per mole) (terbium) | lowest | 2.1×10^-10 m^3/mol (cubic meters per mole) (lutetium) | distribution | Curie point | median | 60 K (kelvins) | highest | 292 K (kelvins) (gadolinium) | lowest | 20 K (kelvins) (holmium) | distribution | Néel point | median | 90.5 K (kelvins) | highest | 230 K (kelvins) (terbium) | lowest | 12.5 K (kelvins) (cerium) | distribution | work function | all | 2.5 eV | 2.7 eV | 2.9 eV | 3 eV | 3.1 eV | 3.2 eV | 3.3 eV | 3.5 eV superconducting point | median | 0.7 K (kelvins) | highest | 4.88 K (kelvins) (lanthanum) | lowest | 0.022 K (kelvins) (cerium) | distribution | color | all |
Reactivity
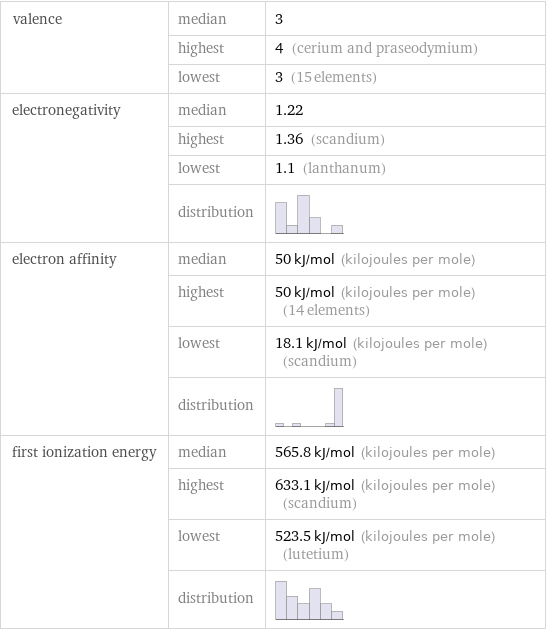
valence | median | 3 | highest | 4 (cerium and praseodymium) | lowest | 3 (15 elements) electronegativity | median | 1.22 | highest | 1.36 (scandium) | lowest | 1.1 (lanthanum) | distribution | electron affinity | median | 50 kJ/mol (kilojoules per mole) | highest | 50 kJ/mol (kilojoules per mole) (14 elements) | lowest | 18.1 kJ/mol (kilojoules per mole) (scandium) | distribution | first ionization energy | median | 565.8 kJ/mol (kilojoules per mole) | highest | 633.1 kJ/mol (kilojoules per mole) (scandium) | lowest | 523.5 kJ/mol (kilojoules per mole) (lutetium) | distribution |
Atomic properties
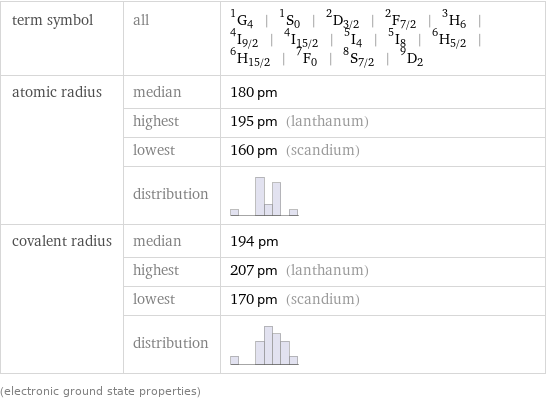
term symbol | all | ^1G_4 | ^1S_0 | ^2D_(3/2) | ^2F_(7/2) | ^3H_6 | ^4I_(9/2) | ^4I_(15/2) | ^5I_4 | ^5I_8 | ^6H_(5/2) | ^6H_(15/2) | ^7F_0 | ^8S_(7/2) | ^9D_2 atomic radius | median | 180 pm | highest | 195 pm (lanthanum) | lowest | 160 pm (scandium) | distribution | covalent radius | median | 194 pm | highest | 207 pm (lanthanum) | lowest | 170 pm (scandium) | distribution | (electronic ground state properties)