Input interpretation
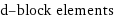
d-block elements
Members

scandium | titanium | vanadium | chromium | manganese | iron | cobalt | nickel | copper | zinc | yttrium | zirconium | niobium | molybdenum | technetium | ruthenium | rhodium | palladium | silver | cadmium | lutetium | hafnium | tantalum | tungsten | rhenium | osmium | iridium | platinum | gold | mercury | lawrencium | rutherfordium | dubnium | seaborgium | bohrium | hassium | meitnerium | darmstadtium | roentgenium | copernicium (total: 40)
Periodic table location
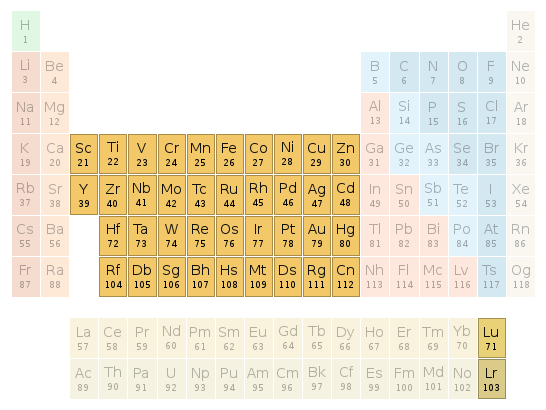
Periodic table location
Images
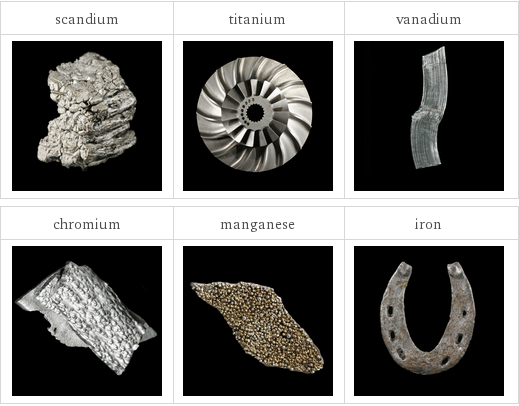
Images
Basic elemental properties
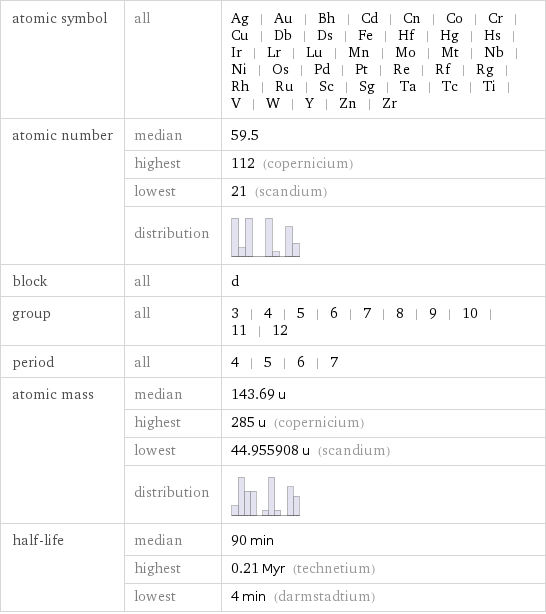
atomic symbol | all | Ag | Au | Bh | Cd | Cn | Co | Cr | Cu | Db | Ds | Fe | Hf | Hg | Hs | Ir | Lr | Lu | Mn | Mo | Mt | Nb | Ni | Os | Pd | Pt | Re | Rf | Rg | Rh | Ru | Sc | Sg | Ta | Tc | Ti | V | W | Y | Zn | Zr atomic number | median | 59.5 | highest | 112 (copernicium) | lowest | 21 (scandium) | distribution | block | all | d group | all | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 period | all | 4 | 5 | 6 | 7 atomic mass | median | 143.69 u | highest | 285 u (copernicium) | lowest | 44.955908 u (scandium) | distribution | half-life | median | 90 min | highest | 0.21 Myr (technetium) | lowest | 4 min (darmstadtium)
Thermodynamic properties
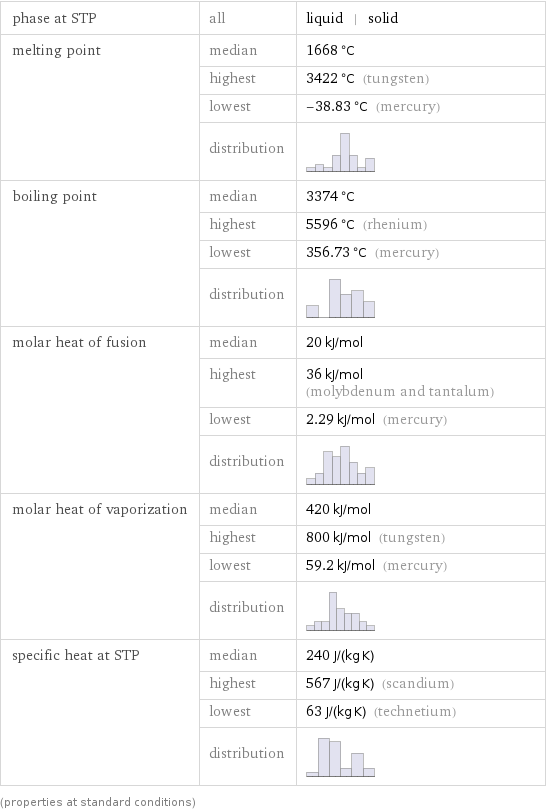
phase at STP | all | liquid | solid melting point | median | 1668 °C | highest | 3422 °C (tungsten) | lowest | -38.83 °C (mercury) | distribution | boiling point | median | 3374 °C | highest | 5596 °C (rhenium) | lowest | 356.73 °C (mercury) | distribution | molar heat of fusion | median | 20 kJ/mol | highest | 36 kJ/mol (molybdenum and tantalum) | lowest | 2.29 kJ/mol (mercury) | distribution | molar heat of vaporization | median | 420 kJ/mol | highest | 800 kJ/mol (tungsten) | lowest | 59.2 kJ/mol (mercury) | distribution | specific heat at STP | median | 240 J/(kg K) | highest | 567 J/(kg K) (scandium) | lowest | 63 J/(kg K) (technetium) | distribution | (properties at standard conditions)
Material properties
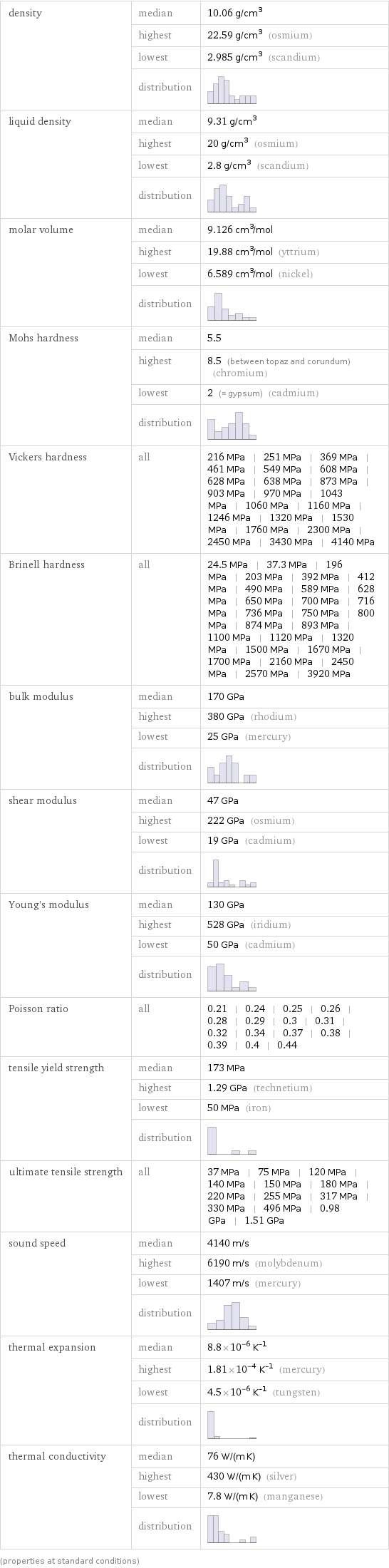
density | median | 10.06 g/cm^3 | highest | 22.59 g/cm^3 (osmium) | lowest | 2.985 g/cm^3 (scandium) | distribution | liquid density | median | 9.31 g/cm^3 | highest | 20 g/cm^3 (osmium) | lowest | 2.8 g/cm^3 (scandium) | distribution | molar volume | median | 9.126 cm^3/mol | highest | 19.88 cm^3/mol (yttrium) | lowest | 6.589 cm^3/mol (nickel) | distribution | Mohs hardness | median | 5.5 | highest | 8.5 (between topaz and corundum) (chromium) | lowest | 2 (≈ gypsum) (cadmium) | distribution | Vickers hardness | all | 216 MPa | 251 MPa | 369 MPa | 461 MPa | 549 MPa | 608 MPa | 628 MPa | 638 MPa | 873 MPa | 903 MPa | 970 MPa | 1043 MPa | 1060 MPa | 1160 MPa | 1246 MPa | 1320 MPa | 1530 MPa | 1760 MPa | 2300 MPa | 2450 MPa | 3430 MPa | 4140 MPa Brinell hardness | all | 24.5 MPa | 37.3 MPa | 196 MPa | 203 MPa | 392 MPa | 412 MPa | 490 MPa | 589 MPa | 628 MPa | 650 MPa | 700 MPa | 716 MPa | 736 MPa | 750 MPa | 800 MPa | 874 MPa | 893 MPa | 1100 MPa | 1120 MPa | 1320 MPa | 1500 MPa | 1670 MPa | 1700 MPa | 2160 MPa | 2450 MPa | 2570 MPa | 3920 MPa bulk modulus | median | 170 GPa | highest | 380 GPa (rhodium) | lowest | 25 GPa (mercury) | distribution | shear modulus | median | 47 GPa | highest | 222 GPa (osmium) | lowest | 19 GPa (cadmium) | distribution | Young's modulus | median | 130 GPa | highest | 528 GPa (iridium) | lowest | 50 GPa (cadmium) | distribution | Poisson ratio | all | 0.21 | 0.24 | 0.25 | 0.26 | 0.28 | 0.29 | 0.3 | 0.31 | 0.32 | 0.34 | 0.37 | 0.38 | 0.39 | 0.4 | 0.44 tensile yield strength | median | 173 MPa | highest | 1.29 GPa (technetium) | lowest | 50 MPa (iron) | distribution | ultimate tensile strength | all | 37 MPa | 75 MPa | 120 MPa | 140 MPa | 150 MPa | 180 MPa | 220 MPa | 255 MPa | 317 MPa | 330 MPa | 496 MPa | 0.98 GPa | 1.51 GPa sound speed | median | 4140 m/s | highest | 6190 m/s (molybdenum) | lowest | 1407 m/s (mercury) | distribution | thermal expansion | median | 8.8×10^-6 K^(-1) | highest | 1.81×10^-4 K^(-1) (mercury) | lowest | 4.5×10^-6 K^(-1) (tungsten) | distribution | thermal conductivity | median | 76 W/(m K) | highest | 430 W/(m K) (silver) | lowest | 7.8 W/(m K) (manganese) | distribution | (properties at standard conditions)
Electromagnetic properties
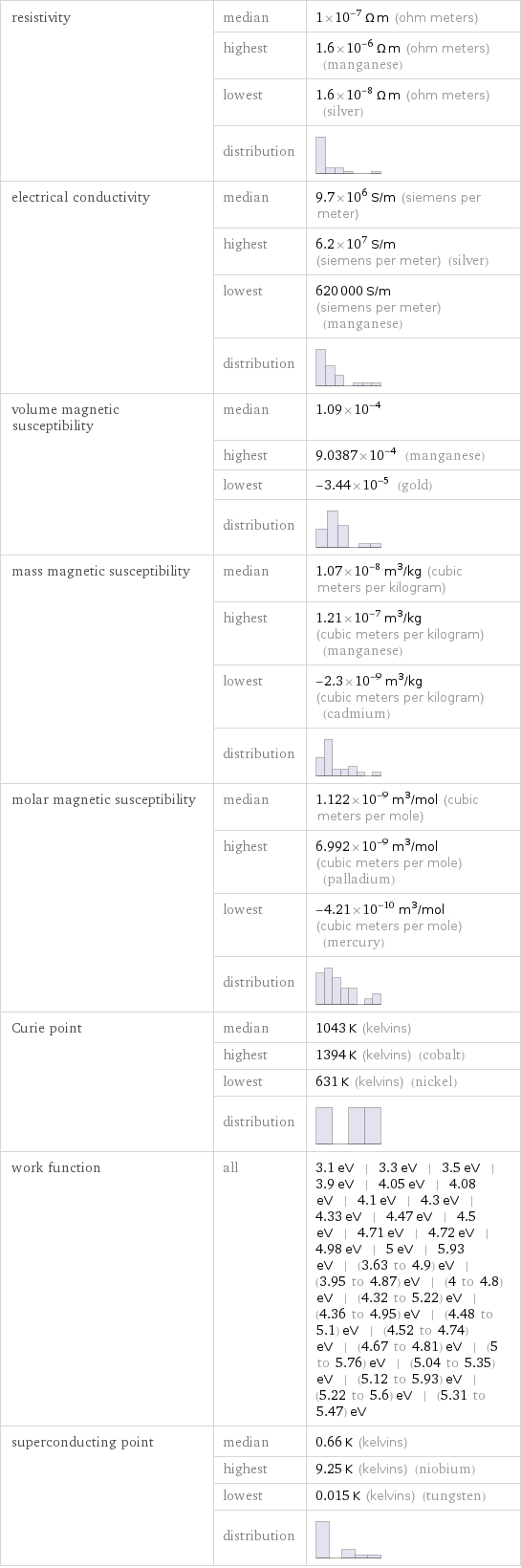
resistivity | median | 1×10^-7 Ω m (ohm meters) | highest | 1.6×10^-6 Ω m (ohm meters) (manganese) | lowest | 1.6×10^-8 Ω m (ohm meters) (silver) | distribution | electrical conductivity | median | 9.7×10^6 S/m (siemens per meter) | highest | 6.2×10^7 S/m (siemens per meter) (silver) | lowest | 620000 S/m (siemens per meter) (manganese) | distribution | volume magnetic susceptibility | median | 1.09×10^-4 | highest | 9.0387×10^-4 (manganese) | lowest | -3.44×10^-5 (gold) | distribution | mass magnetic susceptibility | median | 1.07×10^-8 m^3/kg (cubic meters per kilogram) | highest | 1.21×10^-7 m^3/kg (cubic meters per kilogram) (manganese) | lowest | -2.3×10^-9 m^3/kg (cubic meters per kilogram) (cadmium) | distribution | molar magnetic susceptibility | median | 1.122×10^-9 m^3/mol (cubic meters per mole) | highest | 6.992×10^-9 m^3/mol (cubic meters per mole) (palladium) | lowest | -4.21×10^-10 m^3/mol (cubic meters per mole) (mercury) | distribution | Curie point | median | 1043 K (kelvins) | highest | 1394 K (kelvins) (cobalt) | lowest | 631 K (kelvins) (nickel) | distribution | work function | all | 3.1 eV | 3.3 eV | 3.5 eV | 3.9 eV | 4.05 eV | 4.08 eV | 4.1 eV | 4.3 eV | 4.33 eV | 4.47 eV | 4.5 eV | 4.71 eV | 4.72 eV | 4.98 eV | 5 eV | 5.93 eV | (3.63 to 4.9) eV | (3.95 to 4.87) eV | (4 to 4.8) eV | (4.32 to 5.22) eV | (4.36 to 4.95) eV | (4.48 to 5.1) eV | (4.52 to 4.74) eV | (4.67 to 4.81) eV | (5 to 5.76) eV | (5.04 to 5.35) eV | (5.12 to 5.93) eV | (5.22 to 5.6) eV | (5.31 to 5.47) eV superconducting point | median | 0.66 K (kelvins) | highest | 9.25 K (kelvins) (niobium) | lowest | 0.015 K (kelvins) (tungsten) | distribution |
Reactivity
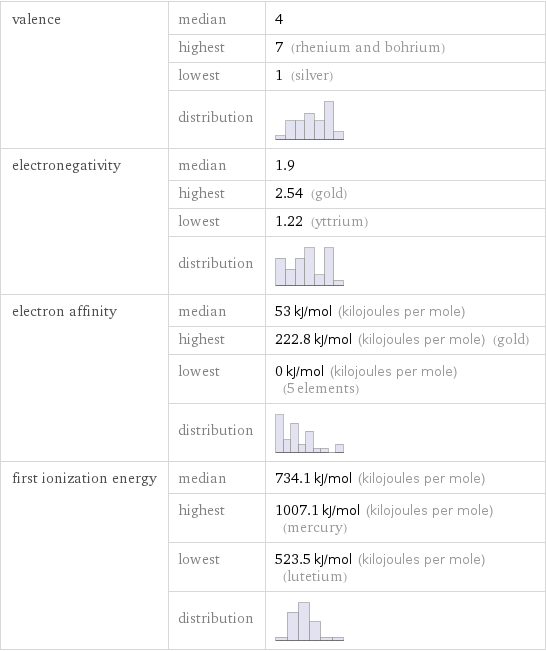
valence | median | 4 | highest | 7 (rhenium and bohrium) | lowest | 1 (silver) | distribution | electronegativity | median | 1.9 | highest | 2.54 (gold) | lowest | 1.22 (yttrium) | distribution | electron affinity | median | 53 kJ/mol (kilojoules per mole) | highest | 222.8 kJ/mol (kilojoules per mole) (gold) | lowest | 0 kJ/mol (kilojoules per mole) (5 elements) | distribution | first ionization energy | median | 734.1 kJ/mol (kilojoules per mole) | highest | 1007.1 kJ/mol (kilojoules per mole) (mercury) | lowest | 523.5 kJ/mol (kilojoules per mole) (lutetium) | distribution |
Atomic properties
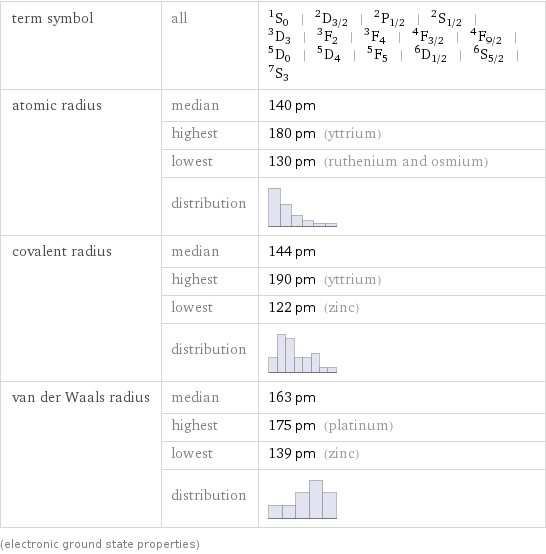
term symbol | all | ^1S_0 | ^2D_(3/2) | ^2P_(1/2) | ^2S_(1/2) | ^3D_3 | ^3F_2 | ^3F_4 | ^4F_(3/2) | ^4F_(9/2) | ^5D_0 | ^5D_4 | ^5F_5 | ^6D_(1/2) | ^6S_(5/2) | ^7S_3 atomic radius | median | 140 pm | highest | 180 pm (yttrium) | lowest | 130 pm (ruthenium and osmium) | distribution | covalent radius | median | 144 pm | highest | 190 pm (yttrium) | lowest | 122 pm (zinc) | distribution | van der Waals radius | median | 163 pm | highest | 175 pm (platinum) | lowest | 139 pm (zinc) | distribution | (electronic ground state properties)