Input interpretation
ethylene oxide
Chemical names and formulas
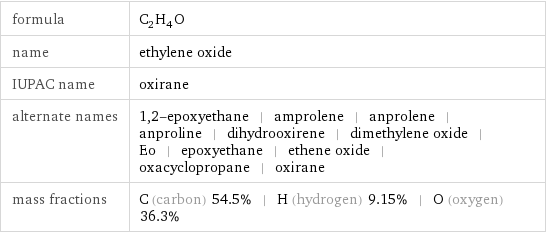
formula | C_2H_4O name | ethylene oxide IUPAC name | oxirane alternate names | 1, 2-epoxyethane | amprolene | anprolene | anproline | dihydrooxirene | dimethylene oxide | Eo | epoxyethane | ethene oxide | oxacyclopropane | oxirane mass fractions | C (carbon) 54.5% | H (hydrogen) 9.15% | O (oxygen) 36.3%
Lewis structure
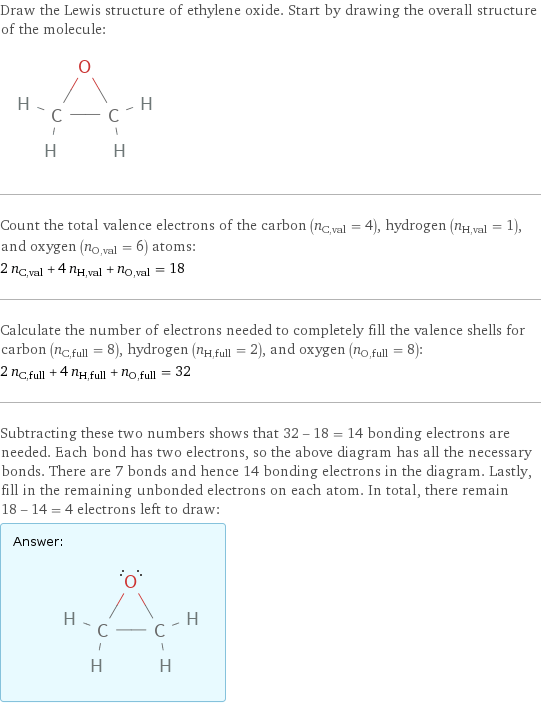
Draw the Lewis structure of ethylene oxide. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms: 2 n_C, val + 4 n_H, val + n_O, val = 18 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): 2 n_C, full + 4 n_H, full + n_O, full = 32 Subtracting these two numbers shows that 32 - 18 = 14 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 7 bonds and hence 14 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 18 - 14 = 4 electrons left to draw: Answer: | |
3D structure

3D structure
Basic properties
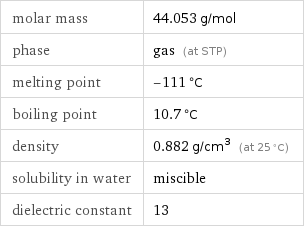
molar mass | 44.053 g/mol phase | gas (at STP) melting point | -111 °C boiling point | 10.7 °C density | 0.882 g/cm^3 (at 25 °C) solubility in water | miscible dielectric constant | 13
Gas properties (at STP)
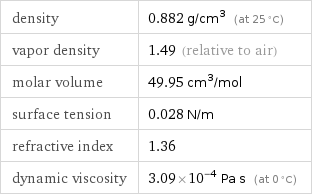
density | 0.882 g/cm^3 (at 25 °C) vapor density | 1.49 (relative to air) molar volume | 49.95 cm^3/mol surface tension | 0.028 N/m refractive index | 1.36 dynamic viscosity | 3.09×10^-4 Pa s (at 0 °C)
Units

Thermodynamic properties
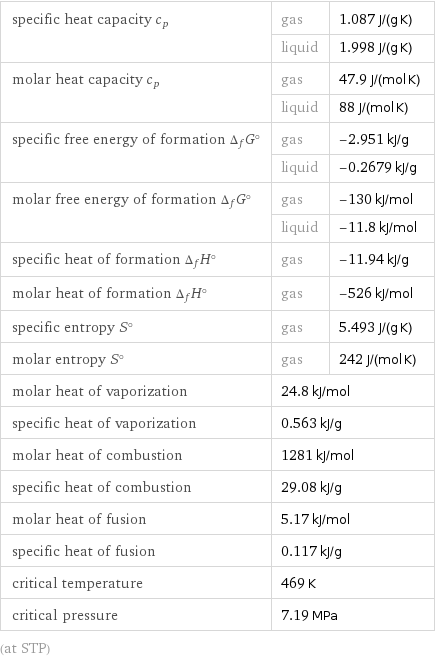
specific heat capacity c_p | gas | 1.087 J/(g K) | liquid | 1.998 J/(g K) molar heat capacity c_p | gas | 47.9 J/(mol K) | liquid | 88 J/(mol K) specific free energy of formation Δ_fG° | gas | -2.951 kJ/g | liquid | -0.2679 kJ/g molar free energy of formation Δ_fG° | gas | -130 kJ/mol | liquid | -11.8 kJ/mol specific heat of formation Δ_fH° | gas | -11.94 kJ/g molar heat of formation Δ_fH° | gas | -526 kJ/mol specific entropy S° | gas | 5.493 J/(g K) molar entropy S° | gas | 242 J/(mol K) molar heat of vaporization | 24.8 kJ/mol | specific heat of vaporization | 0.563 kJ/g | molar heat of combustion | 1281 kJ/mol | specific heat of combustion | 29.08 kJ/g | molar heat of fusion | 5.17 kJ/mol | specific heat of fusion | 0.117 kJ/g | critical temperature | 469 K | critical pressure | 7.19 MPa | (at STP)
Chemical identifiers
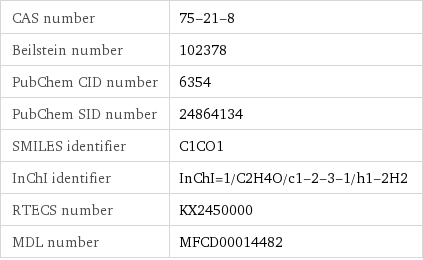
CAS number | 75-21-8 Beilstein number | 102378 PubChem CID number | 6354 PubChem SID number | 24864134 SMILES identifier | C1CO1 InChI identifier | InChI=1/C2H4O/c1-2-3-1/h1-2H2 RTECS number | KX2450000 MDL number | MFCD00014482
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 4 NFPA reactivity rating | 3
Safety properties
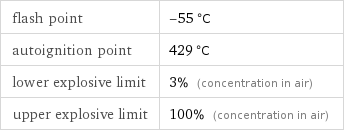
flash point | -55 °C autoignition point | 429 °C lower explosive limit | 3% (concentration in air) upper explosive limit | 100% (concentration in air)
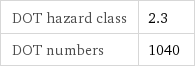
DOT hazard class | 2.3 DOT numbers | 1040
Toxicity properties
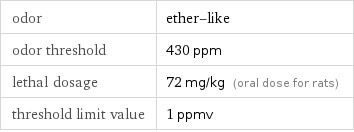
odor | ether-like odor threshold | 430 ppm lethal dosage | 72 mg/kg (oral dose for rats) threshold limit value | 1 ppmv

probable lethal dose for man | 30 mL (milliliters) long-term exposure limit | 10 mg/m^3 (over 8 hours) RTECS classes | agricultural chemical and pesticide | tumorigen | mutagen | reproductive effector | human data | primary irritant