Input interpretation
sodium amide
Chemical names and formulas
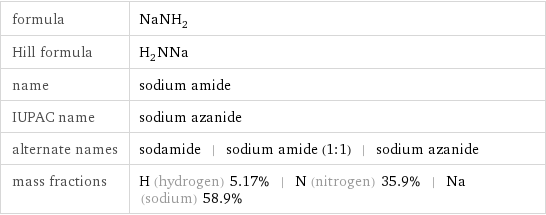
formula | NaNH_2 Hill formula | H_2NNa name | sodium amide IUPAC name | sodium azanide alternate names | sodamide | sodium amide (1:1) | sodium azanide mass fractions | H (hydrogen) 5.17% | N (nitrogen) 35.9% | Na (sodium) 58.9%
Structure diagram
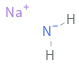
Structure diagram
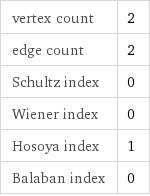
vertex count | 2 edge count | 2 Schultz index | 0 Wiener index | 0 Hosoya index | 1 Balaban index | 0
Basic properties
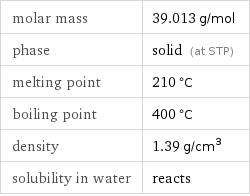
molar mass | 39.013 g/mol phase | solid (at STP) melting point | 210 °C boiling point | 400 °C density | 1.39 g/cm^3 solubility in water | reacts
Units

Solid properties (at STP)

density | 1.39 g/cm^3
Units

Thermodynamic properties
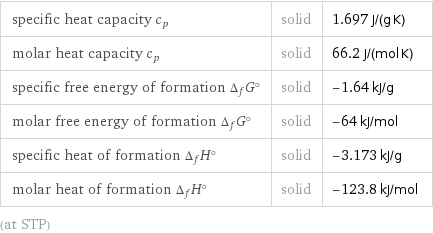
specific heat capacity c_p | solid | 1.697 J/(g K) molar heat capacity c_p | solid | 66.2 J/(mol K) specific free energy of formation Δ_fG° | solid | -1.64 kJ/g molar free energy of formation Δ_fG° | solid | -64 kJ/mol specific heat of formation Δ_fH° | solid | -3.173 kJ/g molar heat of formation Δ_fH° | solid | -123.8 kJ/mol (at STP)
Chemical identifiers
![CAS number | 7782-92-5 PubChem CID number | 24533 PubChem SID number | 24857541 SMILES identifier | [NH2-].[Na+] InChI identifier | InChI=1/H2N.Na/h1H2;/q-1;+1 MDL number | MFCD00011117](../image_source/5e963fa7f7ebbd130a51c84461104728.png)
CAS number | 7782-92-5 PubChem CID number | 24533 PubChem SID number | 24857541 SMILES identifier | [NH2-].[Na+] InChI identifier | InChI=1/H2N.Na/h1H2;/q-1;+1 MDL number | MFCD00011117
NFPA label

NFPA label
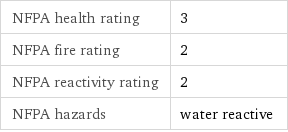
NFPA health rating | 3 NFPA fire rating | 2 NFPA reactivity rating | 2 NFPA hazards | water reactive
Safety properties

flash point | 4.444 °C autoignition point | 450 °C
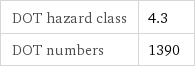
DOT hazard class | 4.3 DOT numbers | 1390
Ion equivalents

Na^+ (sodium cation) | 1 (NH_2)^- (amide anion) | 1