Input interpretation
pyrite | pentaamminechloroiridium(III) chloride
Chemical names and formulas
![| pyrite | pentaamminechloroiridium(III) chloride formula | FeS_2 | [Ir(NH_3)_5Cl]Cl_2 Hill formula | FeS_2 | Cl_3H_15IrN_5 name | pyrite | pentaamminechloroiridium(III) chloride IUPAC name | bis(sulfanylidene)iron | ammonia; trichloroiridium alternate names | iron disulfide | iron pyrite | iron sulfide | ammonia; trichloroiridium mass fractions | Fe (iron) 46.6% | S (sulfur) 53.4% | Cl (chlorine) 27.7% | H (hydrogen) 3.94% | Ir (iridium) 50.1% | N (nitrogen) 18.3%](../image_source/23f0219cfab1d2df5fc21bfc83e8fce1.png)
| pyrite | pentaamminechloroiridium(III) chloride formula | FeS_2 | [Ir(NH_3)_5Cl]Cl_2 Hill formula | FeS_2 | Cl_3H_15IrN_5 name | pyrite | pentaamminechloroiridium(III) chloride IUPAC name | bis(sulfanylidene)iron | ammonia; trichloroiridium alternate names | iron disulfide | iron pyrite | iron sulfide | ammonia; trichloroiridium mass fractions | Fe (iron) 46.6% | S (sulfur) 53.4% | Cl (chlorine) 27.7% | H (hydrogen) 3.94% | Ir (iridium) 50.1% | N (nitrogen) 18.3%
Structure diagrams
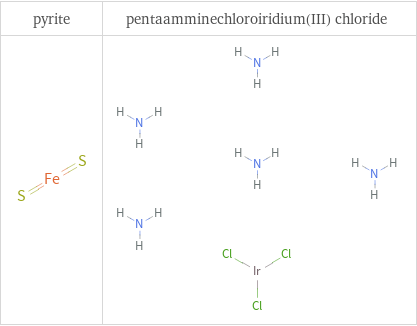
Structure diagrams
Basic properties
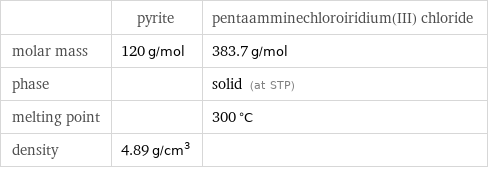
| pyrite | pentaamminechloroiridium(III) chloride molar mass | 120 g/mol | 383.7 g/mol phase | | solid (at STP) melting point | | 300 °C density | 4.89 g/cm^3 |
Units
