Input interpretation
phosphorus tribromide | hassium
Chemical names and formulas
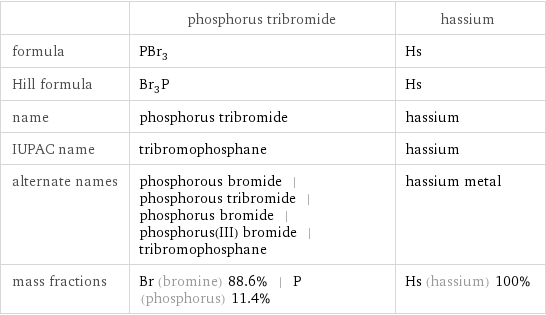
| phosphorus tribromide | hassium formula | PBr_3 | Hs Hill formula | Br_3P | Hs name | phosphorus tribromide | hassium IUPAC name | tribromophosphane | hassium alternate names | phosphorous bromide | phosphorous tribromide | phosphorus bromide | phosphorus(III) bromide | tribromophosphane | hassium metal mass fractions | Br (bromine) 88.6% | P (phosphorus) 11.4% | Hs (hassium) 100%
Structure diagrams

Structure diagrams
3D structure
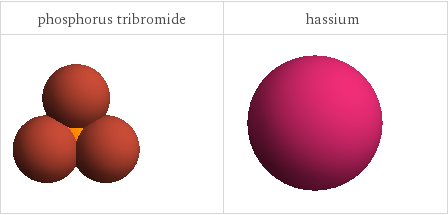
3D structure
Basic properties
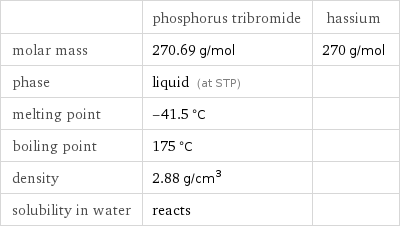
| phosphorus tribromide | hassium molar mass | 270.69 g/mol | 270 g/mol phase | liquid (at STP) | melting point | -41.5 °C | boiling point | 175 °C | density | 2.88 g/cm^3 | solubility in water | reacts |
Units

Liquid properties
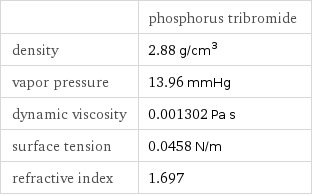
| phosphorus tribromide density | 2.88 g/cm^3 vapor pressure | 13.96 mmHg dynamic viscosity | 0.001302 Pa s surface tension | 0.0458 N/m refractive index | 1.697
Units

Thermodynamic properties
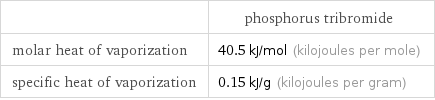
| phosphorus tribromide molar heat of vaporization | 40.5 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.15 kJ/g (kilojoules per gram)