Input interpretation

water | proton count
Result
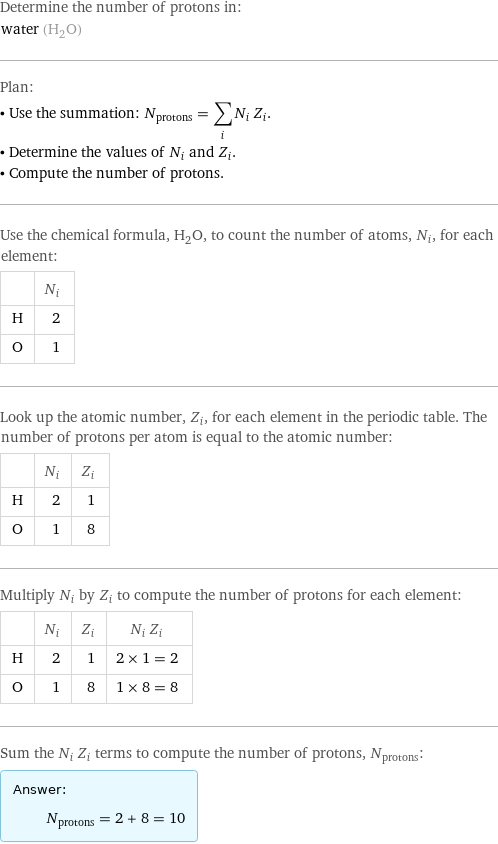
Determine the number of protons in: water (H_2O) Plan: • Use the summation: N_protons = sum_i N_i Z_i. • Determine the values of N_i and Z_i. • Compute the number of protons. Use the chemical formula, H_2O, to count the number of atoms, N_i, for each element: | N_i H | 2 O | 1 Look up the atomic number, Z_i, for each element in the periodic table. The number of protons per atom is equal to the atomic number: | N_i | Z_i H | 2 | 1 O | 1 | 8 Multiply N_i by Z_i to compute the number of protons for each element: | N_i | Z_i | N_i Z_i H | 2 | 1 | 2 × 1 = 2 O | 1 | 8 | 1 × 8 = 8 Sum the N_i Z_i terms to compute the number of protons, N_protons: Answer: | | N_protons = 2 + 8 = 10
Particle counts
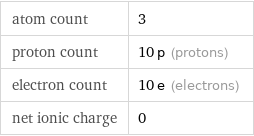
atom count | 3 proton count | 10 p (protons) electron count | 10 e (electrons) net ionic charge | 0
Corresponding quantity
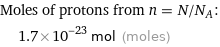
Moles of protons from n = N/N_A: | 1.7×10^-23 mol (moles)