Input interpretation

aspartame
Chemical names and formulas
![formula | C_14H_18N_2O_5 name | aspartame IUPAC name | (3S)-3-azaniumyl-3-[[(1S)-1-methoxycarbonyl-2-phenyl-ethyl]carbamoyl]propanoate alternate names | nutrasweet mass fractions | C (carbon) 57.1% | H (hydrogen) 6.16% | N (nitrogen) 9.52% | O (oxygen) 27.2%](../image_source/b1fa26e2a7f60a2f38fb4c5b73ffd047.png)
formula | C_14H_18N_2O_5 name | aspartame IUPAC name | (3S)-3-azaniumyl-3-[[(1S)-1-methoxycarbonyl-2-phenyl-ethyl]carbamoyl]propanoate alternate names | nutrasweet mass fractions | C (carbon) 57.1% | H (hydrogen) 6.16% | N (nitrogen) 9.52% | O (oxygen) 27.2%
Lewis structure
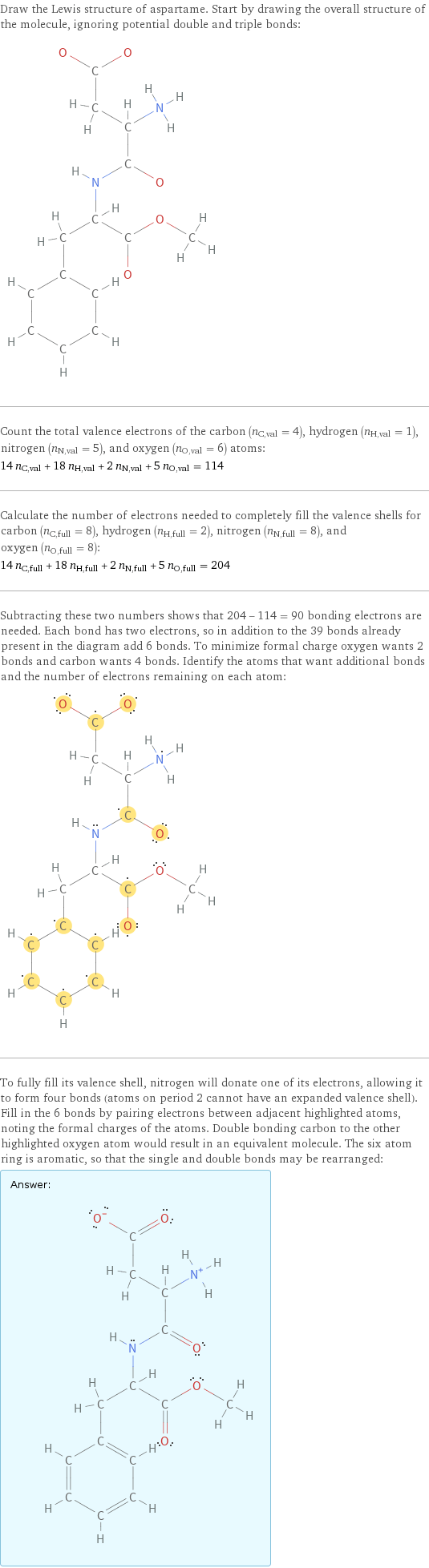
Draw the Lewis structure of aspartame. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 14 n_C, val + 18 n_H, val + 2 n_N, val + 5 n_O, val = 114 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 14 n_C, full + 18 n_H, full + 2 n_N, full + 5 n_O, full = 204 Subtracting these two numbers shows that 204 - 114 = 90 bonding electrons are needed. Each bond has two electrons, so in addition to the 39 bonds already present in the diagram add 6 bonds. To minimize formal charge oxygen wants 2 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: To fully fill its valence shell, nitrogen will donate one of its electrons, allowing it to form four bonds (atoms on period 2 cannot have an expanded valence shell). Fill in the 6 bonds by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms. Double bonding carbon to the other highlighted oxygen atom would result in an equivalent molecule. The six atom ring is aromatic, so that the single and double bonds may be rearranged: Answer: | |
3D structure
3D structure
Basic properties
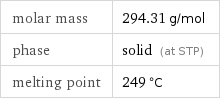
molar mass | 294.31 g/mol phase | solid (at STP) melting point | 249 °C
Units

Hydrophobicity and permeability properties

experimental LogP hydrophobicity | -0.1 predicted LogP hydrophobicity | -1.18 predicted LogS | -2.65
Basic drug properties

approval status | approved | nutraceutical | small molecule drug categories | dietary supplement | micronutrient | sweetening agent
brand names | canderel | equal | nutrasweet | tri-sweet
Chemical identifiers
![CAS number | 22839-47-0 Beilstein number | 2223850 PubChem CID number | 6992066 PubChem SID number | 173626 SMILES identifier | COC(=O)C(CC1=CC=CC=C1)NC(=O)C(CC(=O)[O-])[NH3+] InChI identifier | InChI=1/C14H18N2O5/c1-21-14(20)11(7-9-5-3-2-4-6-9)16-13(19)10(15)8-12(17)18/h2-6, 10-11H, 7-8, 15H2, 1H3, (H, 16, 19)(H, 17, 18)/t10-, 11-/m0/s1/f/h15-16H InChI key | IAOZJIPTCAWIRG-XQMQJMAZCL EU number | 245-261-3 RTECS number | WM3407000](../image_source/8e0042484cd94a4a5bbcd24c07a43568.png)
CAS number | 22839-47-0 Beilstein number | 2223850 PubChem CID number | 6992066 PubChem SID number | 173626 SMILES identifier | COC(=O)C(CC1=CC=CC=C1)NC(=O)C(CC(=O)[O-])[NH3+] InChI identifier | InChI=1/C14H18N2O5/c1-21-14(20)11(7-9-5-3-2-4-6-9)16-13(19)10(15)8-12(17)18/h2-6, 10-11H, 7-8, 15H2, 1H3, (H, 16, 19)(H, 17, 18)/t10-, 11-/m0/s1/f/h15-16H InChI key | IAOZJIPTCAWIRG-XQMQJMAZCL EU number | 245-261-3 RTECS number | WM3407000
NFPA label

NFPA label

NFPA health rating | 0 NFPA fire rating | 1 NFPA reactivity rating | 0
Safety properties

lower explosive limit | 3% (concentration in air) upper explosive limit | 17.5% (concentration in air)
Toxicity properties

RTECS classes | tumorigen | reproductive effector | human data