Input interpretation

period 3 elements | specific heat at STP
Summary
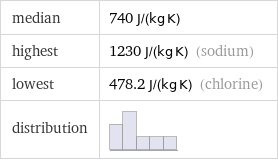
median | 740 J/(kg K) highest | 1230 J/(kg K) (sodium) lowest | 478.2 J/(kg K) (chlorine) distribution |
Units

Ranked values
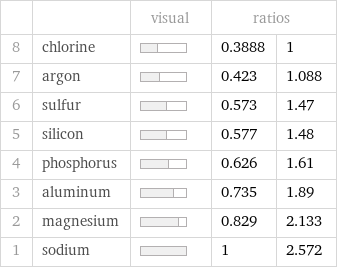
| | visual | ratios | 8 | chlorine | | 0.3888 | 1 7 | argon | | 0.423 | 1.088 6 | sulfur | | 0.573 | 1.47 5 | silicon | | 0.577 | 1.48 4 | phosphorus | | 0.626 | 1.61 3 | aluminum | | 0.735 | 1.89 2 | magnesium | | 0.829 | 2.133 1 | sodium | | 1 | 2.572
Specific heat at STP rankings
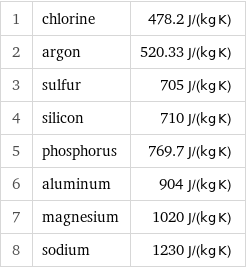
1 | chlorine | 478.2 J/(kg K) 2 | argon | 520.33 J/(kg K) 3 | sulfur | 705 J/(kg K) 4 | silicon | 710 J/(kg K) 5 | phosphorus | 769.7 J/(kg K) 6 | aluminum | 904 J/(kg K) 7 | magnesium | 1020 J/(kg K) 8 | sodium | 1230 J/(kg K)
Unit conversions for median specific heat at STP 740 J/(kg K)
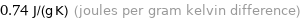
0.74 J/(g K) (joules per gram kelvin difference)
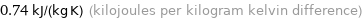
0.74 kJ/(kg K) (kilojoules per kilogram kelvin difference)
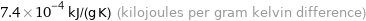
7.4×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

740 J/(kg °C) (joules per kilogram degree Celsius difference)
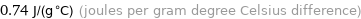
0.74 J/(g °C) (joules per gram degree Celsius difference)

0.177 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons for median specific heat at STP 740 J/(kg K)
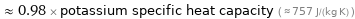
≈ 0.98 × potassium specific heat capacity ( ≈ 757 J/(kg K) )

≈ graphite specific heat capacity ( ≈ 720 J/(kg K) )
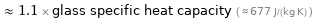
≈ 1.1 × glass specific heat capacity ( ≈ 677 J/(kg K) )
Thermodynamic properties
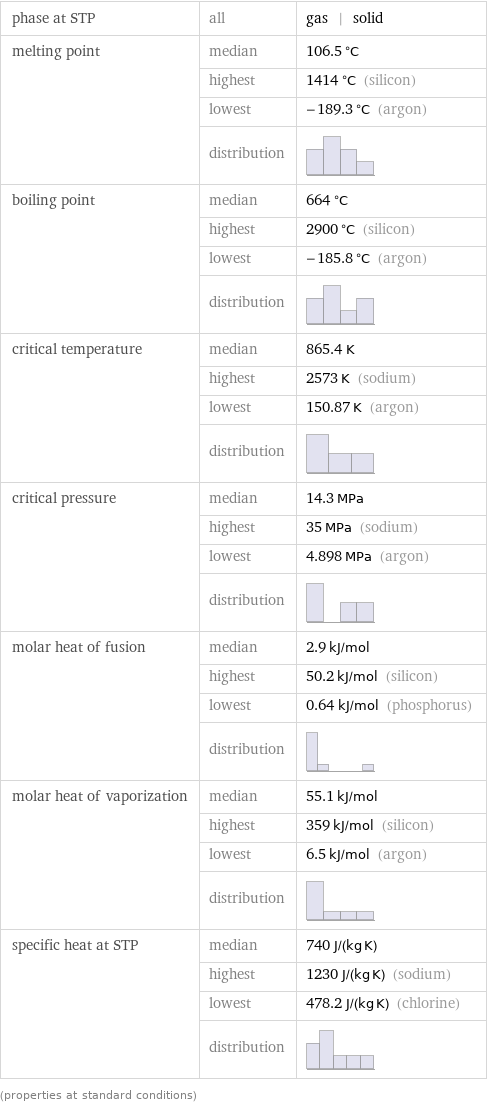
phase at STP | all | gas | solid melting point | median | 106.5 °C | highest | 1414 °C (silicon) | lowest | -189.3 °C (argon) | distribution | boiling point | median | 664 °C | highest | 2900 °C (silicon) | lowest | -185.8 °C (argon) | distribution | critical temperature | median | 865.4 K | highest | 2573 K (sodium) | lowest | 150.87 K (argon) | distribution | critical pressure | median | 14.3 MPa | highest | 35 MPa (sodium) | lowest | 4.898 MPa (argon) | distribution | molar heat of fusion | median | 2.9 kJ/mol | highest | 50.2 kJ/mol (silicon) | lowest | 0.64 kJ/mol (phosphorus) | distribution | molar heat of vaporization | median | 55.1 kJ/mol | highest | 359 kJ/mol (silicon) | lowest | 6.5 kJ/mol (argon) | distribution | specific heat at STP | median | 740 J/(kg K) | highest | 1230 J/(kg K) (sodium) | lowest | 478.2 J/(kg K) (chlorine) | distribution | (properties at standard conditions)