Input interpretation
cations | lanthanide ions
Members
Cations

hydrogen cation | ammonium cation | sodium cation | magnesium cation | aluminum cation | potassium cation | calcium cation | chromium(II) cation | chromium(III) cation | iron(II) cation | iron(III) cation | cobalt(II) cation | cobalt(III) cation | copper(I) cation | copper(II) cation | silver(I) cation | dimercury(I) cation | mercury(II) cation | lead(II) cation | lead(IV) cation (total: 20)
Lanthanide ions

lanthanum(III) cation | cerium(III) cation | cerium(IV) cation | praseodymium(III) cation | neodymium(III) cation | promethium(III) cation | samarium(III) cation | europium(II) cation | europium(III) cation | gadolinium(III) cation | terbium(III) cation | dysprosium(III) cation | holmium(III) cation | erbium(III) cation | thullium(III) cation | ytterbium(III) cation | lutetium(III) cation (total: 17)
Ionic radii
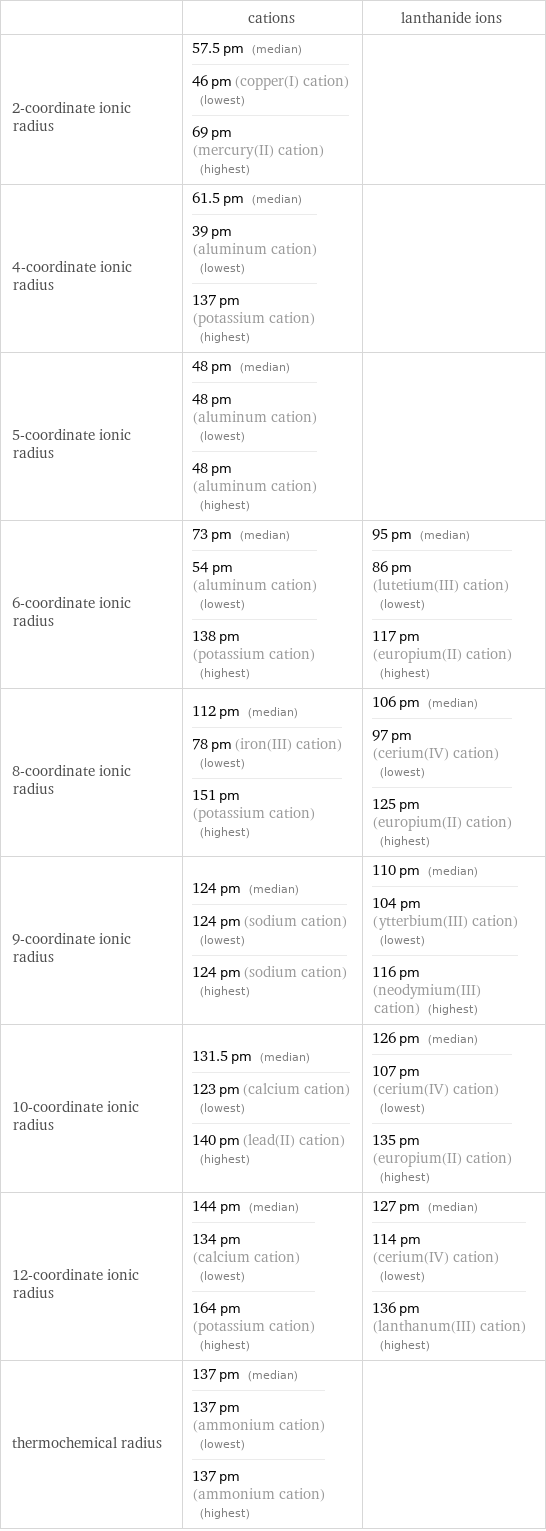
| cations | lanthanide ions 2-coordinate ionic radius | 57.5 pm (median) 46 pm (copper(I) cation) (lowest) 69 pm (mercury(II) cation) (highest) | 4-coordinate ionic radius | 61.5 pm (median) 39 pm (aluminum cation) (lowest) 137 pm (potassium cation) (highest) | 5-coordinate ionic radius | 48 pm (median) 48 pm (aluminum cation) (lowest) 48 pm (aluminum cation) (highest) | 6-coordinate ionic radius | 73 pm (median) 54 pm (aluminum cation) (lowest) 138 pm (potassium cation) (highest) | 95 pm (median) 86 pm (lutetium(III) cation) (lowest) 117 pm (europium(II) cation) (highest) 8-coordinate ionic radius | 112 pm (median) 78 pm (iron(III) cation) (lowest) 151 pm (potassium cation) (highest) | 106 pm (median) 97 pm (cerium(IV) cation) (lowest) 125 pm (europium(II) cation) (highest) 9-coordinate ionic radius | 124 pm (median) 124 pm (sodium cation) (lowest) 124 pm (sodium cation) (highest) | 110 pm (median) 104 pm (ytterbium(III) cation) (lowest) 116 pm (neodymium(III) cation) (highest) 10-coordinate ionic radius | 131.5 pm (median) 123 pm (calcium cation) (lowest) 140 pm (lead(II) cation) (highest) | 126 pm (median) 107 pm (cerium(IV) cation) (lowest) 135 pm (europium(II) cation) (highest) 12-coordinate ionic radius | 144 pm (median) 134 pm (calcium cation) (lowest) 164 pm (potassium cation) (highest) | 127 pm (median) 114 pm (cerium(IV) cation) (lowest) 136 pm (lanthanum(III) cation) (highest) thermochemical radius | 137 pm (median) 137 pm (ammonium cation) (lowest) 137 pm (ammonium cation) (highest) |