Input interpretation
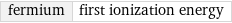
fermium | first ionization energy
Result
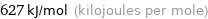
627 kJ/mol (kilojoules per mole)
Periodic table location
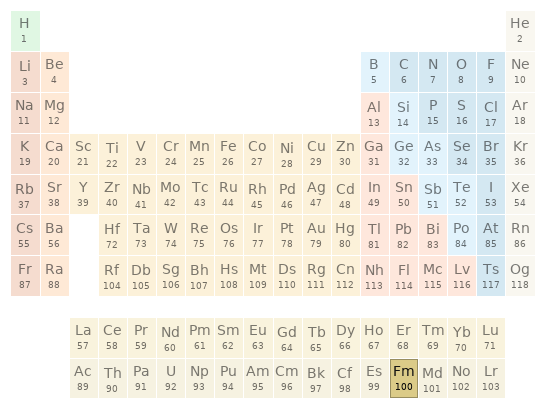
Periodic table location
Unit conversions
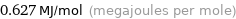
0.627 MJ/mol (megajoules per mole)
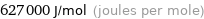
627000 J/mol (joules per mole)
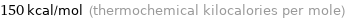
150 kcal/mol (thermochemical kilocalories per mole)
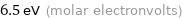
6.5 eV (molar electronvolts)
Comparisons as molar ionization energy

≈ 0.26 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
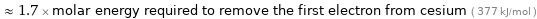
≈ 1.7 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )