Input interpretation
1, 2-diiodoethane | 1-iodo-3-phenylpropane
Chemical names and formulas
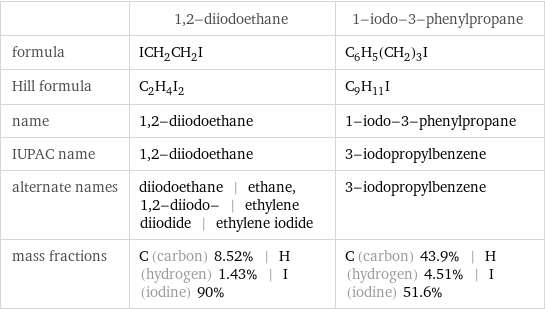
| 1, 2-diiodoethane | 1-iodo-3-phenylpropane formula | ICH_2CH_2I | C_6H_5(CH_2)_3I Hill formula | C_2H_4I_2 | C_9H_11I name | 1, 2-diiodoethane | 1-iodo-3-phenylpropane IUPAC name | 1, 2-diiodoethane | 3-iodopropylbenzene alternate names | diiodoethane | ethane, 1, 2-diiodo- | ethylene diiodide | ethylene iodide | 3-iodopropylbenzene mass fractions | C (carbon) 8.52% | H (hydrogen) 1.43% | I (iodine) 90% | C (carbon) 43.9% | H (hydrogen) 4.51% | I (iodine) 51.6%
Structure diagrams
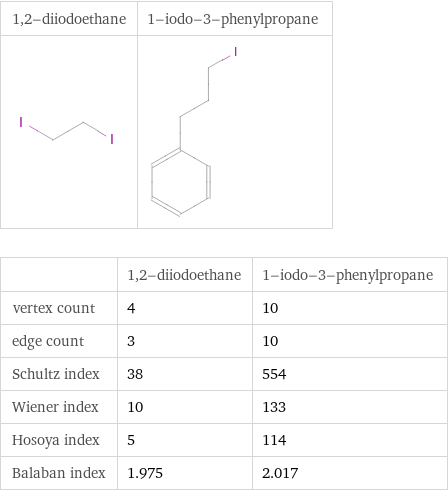
| 1, 2-diiodoethane | 1-iodo-3-phenylpropane vertex count | 4 | 10 edge count | 3 | 10 Schultz index | 38 | 554 Wiener index | 10 | 133 Hosoya index | 5 | 114 Balaban index | 1.975 | 2.017
3D structure
3D structure
Basic properties
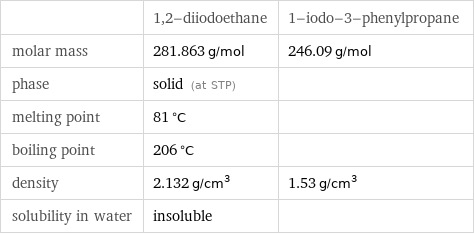
| 1, 2-diiodoethane | 1-iodo-3-phenylpropane molar mass | 281.863 g/mol | 246.09 g/mol phase | solid (at STP) | melting point | 81 °C | boiling point | 206 °C | density | 2.132 g/cm^3 | 1.53 g/cm^3 solubility in water | insoluble |
Units

Thermodynamic properties
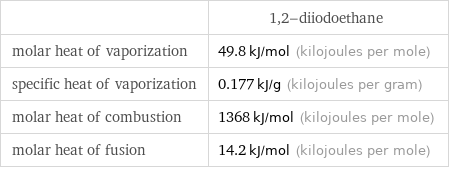
| 1, 2-diiodoethane molar heat of vaporization | 49.8 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.177 kJ/g (kilojoules per gram) molar heat of combustion | 1368 kJ/mol (kilojoules per mole) molar heat of fusion | 14.2 kJ/mol (kilojoules per mole)