Input interpretation

diprotic acids | boiling point
Summary

median | 336 °C highest | 553 °C (ascorbic acid) lowest | -60 °C (hydrogen sulfide) distribution | | (based on 8 values; 7 unavailable)
Entities with missing values
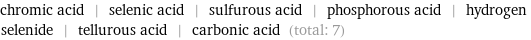
chromic acid | selenic acid | sulfurous acid | phosphorous acid | hydrogen selenide | tellurous acid | carbonic acid (total: 7)
Boiling point rankings
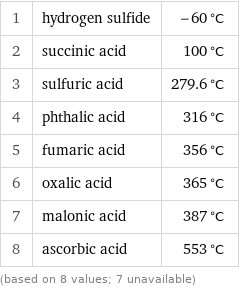
1 | hydrogen sulfide | -60 °C 2 | succinic acid | 100 °C 3 | sulfuric acid | 279.6 °C 4 | phthalic acid | 316 °C 5 | fumaric acid | 356 °C 6 | oxalic acid | 365 °C 7 | malonic acid | 387 °C 8 | ascorbic acid | 553 °C (based on 8 values; 7 unavailable)
Unit conversions for median boiling point 336 °C
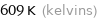
609 K (kelvins)
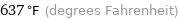
637 °F (degrees Fahrenheit)
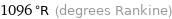
1096 °R (degrees Rankine)

269 °Ré (degrees Réaumur)
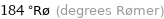
184 °Rø (degrees Rømer)
Comparison for median boiling point 336 °C
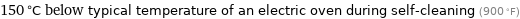
150 °C below typical temperature of an electric oven during self-cleaning (900 °F)
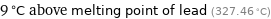
9 °C above melting point of lead (327.46 °C)
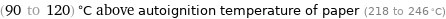
(90 to 120) °C above autoignition temperature of paper (218 to 246 °C)
Corresponding quantities

Thermodynamic energy E from E = kT: | 52 meV (millielectronvolts)
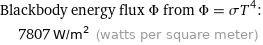
Blackbody energy flux Φ from Φ = σT^4: | 7807 W/m^2 (watts per square meter)

Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 3.7×10^-6 lx (lux)