Input interpretation
beryllium chloride
Chemical names and formulas

formula | BeCl_2 name | beryllium chloride IUPAC name | beryllium dichloride alternate names | beryllium dichloride mass fractions | Be (beryllium) 11.3% | Cl (chlorine) 88.7%
Structure diagram
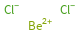
Structure diagram
Basic properties
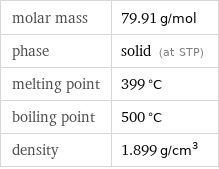
molar mass | 79.91 g/mol phase | solid (at STP) melting point | 399 °C boiling point | 500 °C density | 1.899 g/cm^3
Units

Solid properties (at STP)

density | 1.899 g/cm^3
Units

Thermodynamic properties
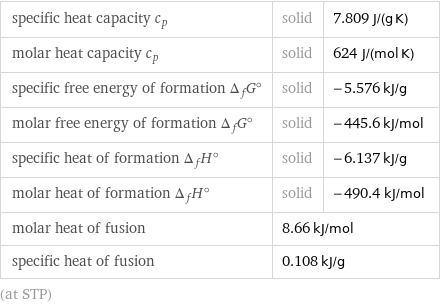
specific heat capacity c_p | solid | 7.809 J/(g K) molar heat capacity c_p | solid | 624 J/(mol K) specific free energy of formation Δ_fG° | solid | -5.576 kJ/g molar free energy of formation Δ_fG° | solid | -445.6 kJ/mol specific heat of formation Δ_fH° | solid | -6.137 kJ/g molar heat of formation Δ_fH° | solid | -490.4 kJ/mol molar heat of fusion | 8.66 kJ/mol | specific heat of fusion | 0.108 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7787-47-5 PubChem CID number | 24588 PubChem SID number | 24852001 SMILES identifier | [Be+2].[Cl-].[Cl-] InChI identifier | InChI=1/Be.2ClH/h;2*1H/q+2;;/p-2/fBe.2Cl/h;2*1h/qm;2*-1 RTECS number | DS2625000 MDL number | MFCD00042674](../image_source/1916ca63cd7ee28c79e01a67e241a3ec.png)
CAS number | 7787-47-5 PubChem CID number | 24588 PubChem SID number | 24852001 SMILES identifier | [Be+2].[Cl-].[Cl-] InChI identifier | InChI=1/Be.2ClH/h;2*1H/q+2;;/p-2/fBe.2Cl/h;2*1h/qm;2*-1 RTECS number | DS2625000 MDL number | MFCD00042674
Toxicity properties

RTECS classes | tumorigen | mutagen | reproductive effector
Ion equivalents

Be^(2+) (beryllium cation) | 1 Cl^- (chloride anion) | 2