Input interpretation
acetylene | 1-methylpyrene
Chemical names and formulas
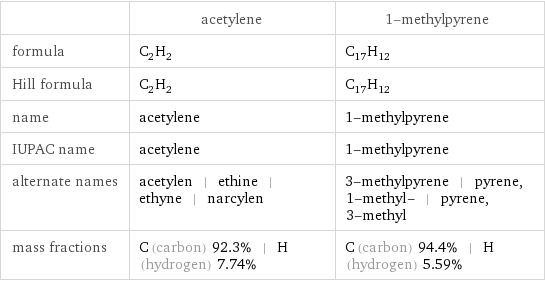
| acetylene | 1-methylpyrene formula | C_2H_2 | C_17H_12 Hill formula | C_2H_2 | C_17H_12 name | acetylene | 1-methylpyrene IUPAC name | acetylene | 1-methylpyrene alternate names | acetylen | ethine | ethyne | narcylen | 3-methylpyrene | pyrene, 1-methyl- | pyrene, 3-methyl mass fractions | C (carbon) 92.3% | H (hydrogen) 7.74% | C (carbon) 94.4% | H (hydrogen) 5.59%
Structure diagrams
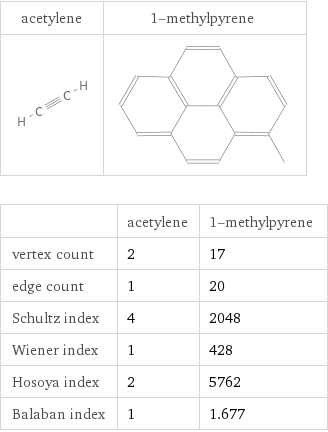
| acetylene | 1-methylpyrene vertex count | 2 | 17 edge count | 1 | 20 Schultz index | 4 | 2048 Wiener index | 1 | 428 Hosoya index | 2 | 5762 Balaban index | 1 | 1.677
3D structure
3D structure
Basic properties
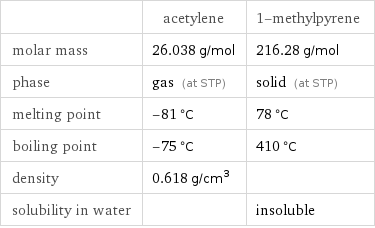
| acetylene | 1-methylpyrene molar mass | 26.038 g/mol | 216.28 g/mol phase | gas (at STP) | solid (at STP) melting point | -81 °C | 78 °C boiling point | -75 °C | 410 °C density | 0.618 g/cm^3 | solubility in water | | insoluble
Units

Gas properties
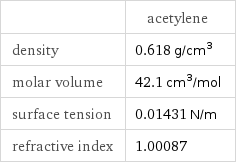
| acetylene density | 0.618 g/cm^3 molar volume | 42.1 cm^3/mol surface tension | 0.01431 N/m refractive index | 1.00087
Units
Thermodynamic properties
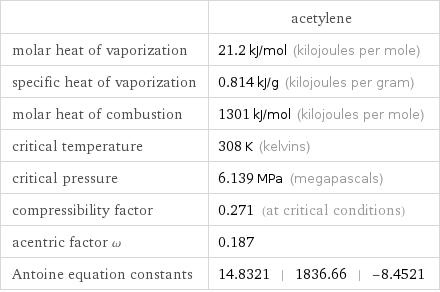
| acetylene molar heat of vaporization | 21.2 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.814 kJ/g (kilojoules per gram) molar heat of combustion | 1301 kJ/mol (kilojoules per mole) critical temperature | 308 K (kelvins) critical pressure | 6.139 MPa (megapascals) compressibility factor | 0.271 (at critical conditions) acentric factor ω | 0.187 Antoine equation constants | 14.8321 | 1836.66 | -8.4521