Input interpretation
magnesium nitride | perchloryl fluoride
Chemical names and formulas
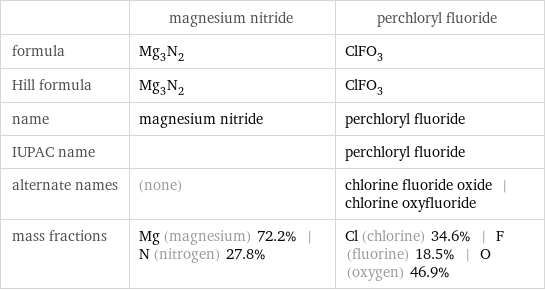
| magnesium nitride | perchloryl fluoride formula | Mg_3N_2 | ClFO_3 Hill formula | Mg_3N_2 | ClFO_3 name | magnesium nitride | perchloryl fluoride IUPAC name | | perchloryl fluoride alternate names | (none) | chlorine fluoride oxide | chlorine oxyfluoride mass fractions | Mg (magnesium) 72.2% | N (nitrogen) 27.8% | Cl (chlorine) 34.6% | F (fluorine) 18.5% | O (oxygen) 46.9%
Structure diagrams
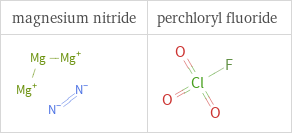
Structure diagrams
Basic properties
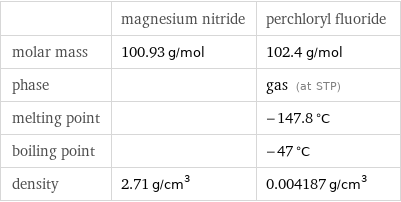
| magnesium nitride | perchloryl fluoride molar mass | 100.93 g/mol | 102.4 g/mol phase | | gas (at STP) melting point | | -147.8 °C boiling point | | -47 °C density | 2.71 g/cm^3 | 0.004187 g/cm^3
Units

Gas properties
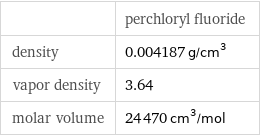
| perchloryl fluoride density | 0.004187 g/cm^3 vapor density | 3.64 molar volume | 24470 cm^3/mol
Units

Thermodynamic properties
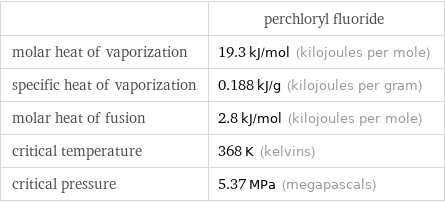
| perchloryl fluoride molar heat of vaporization | 19.3 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.188 kJ/g (kilojoules per gram) molar heat of fusion | 2.8 kJ/mol (kilojoules per mole) critical temperature | 368 K (kelvins) critical pressure | 5.37 MPa (megapascals)