Input interpretation
ferromagnetic elements
Members
cobalt | gadolinium | iron | nickel
Periodic table location
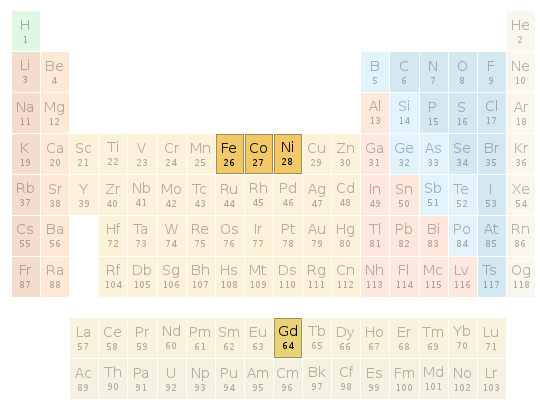
Periodic table location
Images
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration cobalt | Co | 27 | [Ar]4s^23d^7 gadolinium | Gd | 64 | [Xe]6s^24f^75d^1 iron | Fe | 26 | [Ar]4s^23d^6 nickel | Ni | 28 | [Ar]4s^23d^8 | Aufbau diagram | block | group cobalt | 3d 4s | d | 9 gadolinium | 5d 4f 6s | f | iron | 3d 4s | d | 8 nickel | 3d 4s | d | 10 | period | atomic mass | half-life cobalt | 4 | 58.933194 u | (stable) gadolinium | 6 | 157.25 u | (stable) iron | 4 | 55.845 u | (stable) nickel | 4 | 58.6934 u | (stable)](../image_source/bb7fb3033e73effd70e1b563d20d76f6.png)
| atomic symbol | atomic number | short electronic configuration cobalt | Co | 27 | [Ar]4s^23d^7 gadolinium | Gd | 64 | [Xe]6s^24f^75d^1 iron | Fe | 26 | [Ar]4s^23d^6 nickel | Ni | 28 | [Ar]4s^23d^8 | Aufbau diagram | block | group cobalt | 3d 4s | d | 9 gadolinium | 5d 4f 6s | f | iron | 3d 4s | d | 8 nickel | 3d 4s | d | 10 | period | atomic mass | half-life cobalt | 4 | 58.933194 u | (stable) gadolinium | 6 | 157.25 u | (stable) iron | 4 | 55.845 u | (stable) nickel | 4 | 58.6934 u | (stable)
Thermodynamic properties
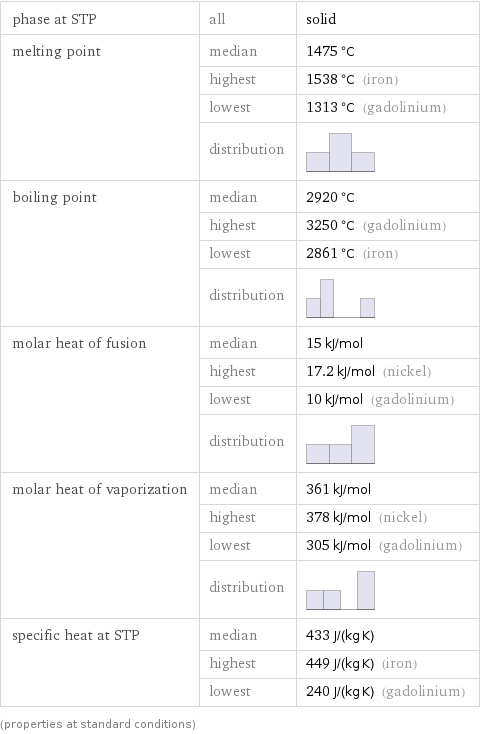
phase at STP | all | solid melting point | median | 1475 °C | highest | 1538 °C (iron) | lowest | 1313 °C (gadolinium) | distribution | boiling point | median | 2920 °C | highest | 3250 °C (gadolinium) | lowest | 2861 °C (iron) | distribution | molar heat of fusion | median | 15 kJ/mol | highest | 17.2 kJ/mol (nickel) | lowest | 10 kJ/mol (gadolinium) | distribution | molar heat of vaporization | median | 361 kJ/mol | highest | 378 kJ/mol (nickel) | lowest | 305 kJ/mol (gadolinium) | distribution | specific heat at STP | median | 433 J/(kg K) | highest | 449 J/(kg K) (iron) | lowest | 240 J/(kg K) (gadolinium) (properties at standard conditions)
Material properties
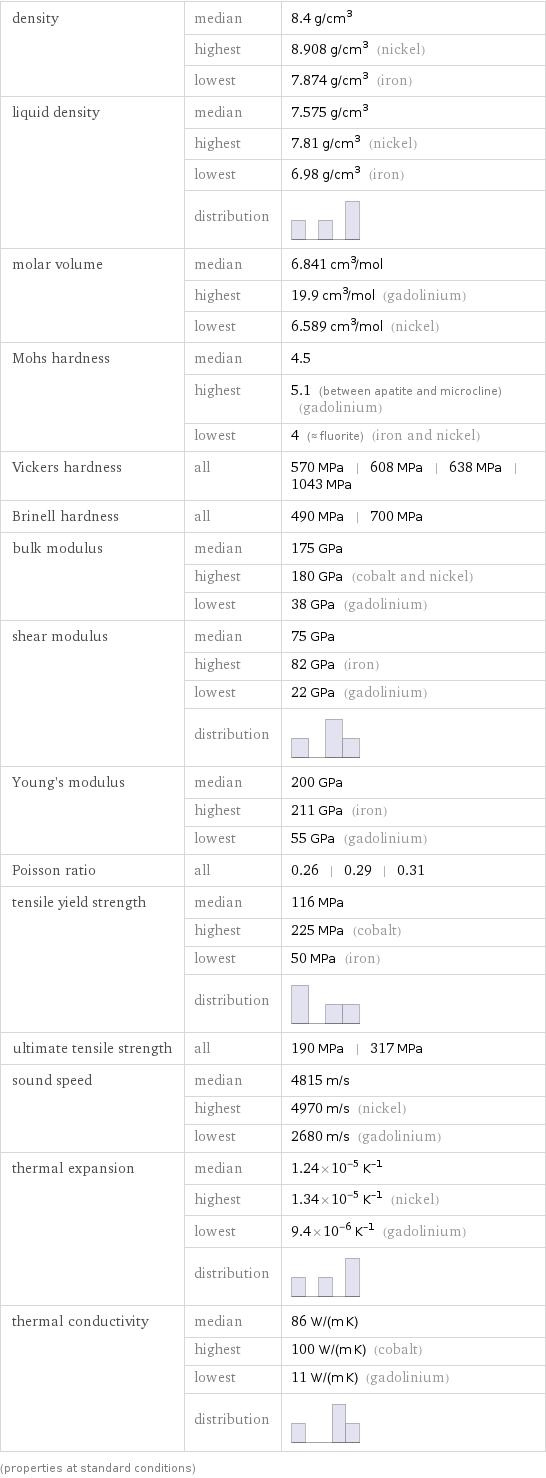
density | median | 8.4 g/cm^3 | highest | 8.908 g/cm^3 (nickel) | lowest | 7.874 g/cm^3 (iron) liquid density | median | 7.575 g/cm^3 | highest | 7.81 g/cm^3 (nickel) | lowest | 6.98 g/cm^3 (iron) | distribution | molar volume | median | 6.841 cm^3/mol | highest | 19.9 cm^3/mol (gadolinium) | lowest | 6.589 cm^3/mol (nickel) Mohs hardness | median | 4.5 | highest | 5.1 (between apatite and microcline) (gadolinium) | lowest | 4 (≈ fluorite) (iron and nickel) Vickers hardness | all | 570 MPa | 608 MPa | 638 MPa | 1043 MPa Brinell hardness | all | 490 MPa | 700 MPa bulk modulus | median | 175 GPa | highest | 180 GPa (cobalt and nickel) | lowest | 38 GPa (gadolinium) shear modulus | median | 75 GPa | highest | 82 GPa (iron) | lowest | 22 GPa (gadolinium) | distribution | Young's modulus | median | 200 GPa | highest | 211 GPa (iron) | lowest | 55 GPa (gadolinium) Poisson ratio | all | 0.26 | 0.29 | 0.31 tensile yield strength | median | 116 MPa | highest | 225 MPa (cobalt) | lowest | 50 MPa (iron) | distribution | ultimate tensile strength | all | 190 MPa | 317 MPa sound speed | median | 4815 m/s | highest | 4970 m/s (nickel) | lowest | 2680 m/s (gadolinium) thermal expansion | median | 1.24×10^-5 K^(-1) | highest | 1.34×10^-5 K^(-1) (nickel) | lowest | 9.4×10^-6 K^(-1) (gadolinium) | distribution | thermal conductivity | median | 86 W/(m K) | highest | 100 W/(m K) (cobalt) | lowest | 11 W/(m K) (gadolinium) | distribution | (properties at standard conditions)
Electromagnetic properties
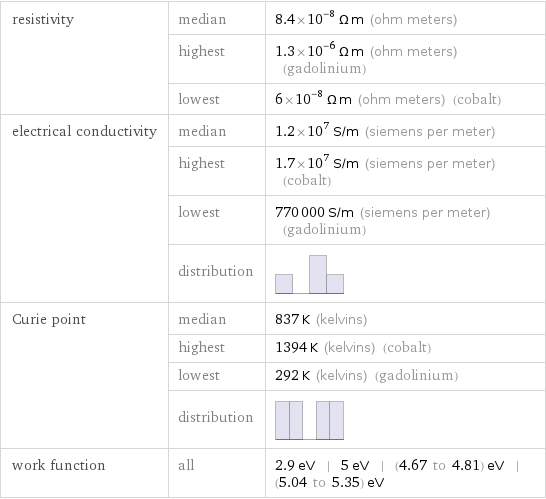
resistivity | median | 8.4×10^-8 Ω m (ohm meters) | highest | 1.3×10^-6 Ω m (ohm meters) (gadolinium) | lowest | 6×10^-8 Ω m (ohm meters) (cobalt) electrical conductivity | median | 1.2×10^7 S/m (siemens per meter) | highest | 1.7×10^7 S/m (siemens per meter) (cobalt) | lowest | 770000 S/m (siemens per meter) (gadolinium) | distribution | Curie point | median | 837 K (kelvins) | highest | 1394 K (kelvins) (cobalt) | lowest | 292 K (kelvins) (gadolinium) | distribution | work function | all | 2.9 eV | 5 eV | (4.67 to 4.81) eV | (5.04 to 5.35) eV
Reactivity
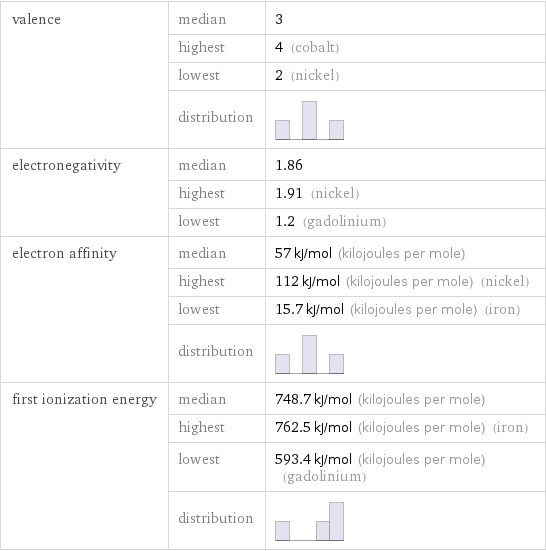
valence | median | 3 | highest | 4 (cobalt) | lowest | 2 (nickel) | distribution | electronegativity | median | 1.86 | highest | 1.91 (nickel) | lowest | 1.2 (gadolinium) electron affinity | median | 57 kJ/mol (kilojoules per mole) | highest | 112 kJ/mol (kilojoules per mole) (nickel) | lowest | 15.7 kJ/mol (kilojoules per mole) (iron) | distribution | first ionization energy | median | 748.7 kJ/mol (kilojoules per mole) | highest | 762.5 kJ/mol (kilojoules per mole) (iron) | lowest | 593.4 kJ/mol (kilojoules per mole) (gadolinium) | distribution |
Atomic properties
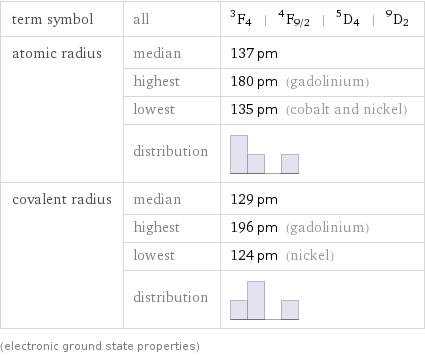
term symbol | all | ^3F_4 | ^4F_(9/2) | ^5D_4 | ^9D_2 atomic radius | median | 137 pm | highest | 180 pm (gadolinium) | lowest | 135 pm (cobalt and nickel) | distribution | covalent radius | median | 129 pm | highest | 196 pm (gadolinium) | lowest | 124 pm (nickel) | distribution | (electronic ground state properties)