Input interpretation
2-chloro-2-methylpropane
Chemical names and formulas
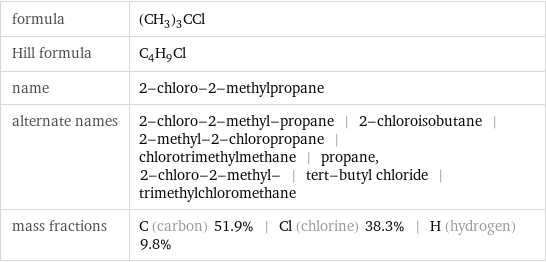
formula | (CH_3)_3CCl Hill formula | C_4H_9Cl name | 2-chloro-2-methylpropane alternate names | 2-chloro-2-methyl-propane | 2-chloroisobutane | 2-methyl-2-chloropropane | chlorotrimethylmethane | propane, 2-chloro-2-methyl- | tert-butyl chloride | trimethylchloromethane mass fractions | C (carbon) 51.9% | Cl (chlorine) 38.3% | H (hydrogen) 9.8%
Lewis structure

Draw the Lewis structure of 2-chloro-2-methylpropane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), and hydrogen (n_H, val = 1) atoms: 4 n_C, val + n_Cl, val + 9 n_H, val = 32 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), and hydrogen (n_H, full = 2): 4 n_C, full + n_Cl, full + 9 n_H, full = 58 Subtracting these two numbers shows that 58 - 32 = 26 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 13 bonds and hence 26 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 32 - 26 = 6 electrons left to draw: Answer: | |
3D structure
3D structure
Basic properties
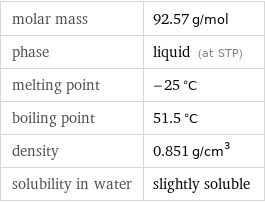
molar mass | 92.57 g/mol phase | liquid (at STP) melting point | -25 °C boiling point | 51.5 °C density | 0.851 g/cm^3 solubility in water | slightly soluble
Units

Liquid properties (at STP)
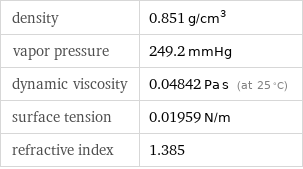
density | 0.851 g/cm^3 vapor pressure | 249.2 mmHg dynamic viscosity | 0.04842 Pa s (at 25 °C) surface tension | 0.01959 N/m refractive index | 1.385
Units
Thermodynamic properties
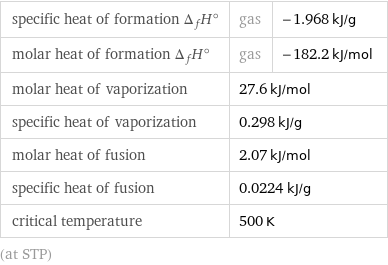
specific heat of formation Δ_fH° | gas | -1.968 kJ/g molar heat of formation Δ_fH° | gas | -182.2 kJ/mol molar heat of vaporization | 27.6 kJ/mol | specific heat of vaporization | 0.298 kJ/g | molar heat of fusion | 2.07 kJ/mol | specific heat of fusion | 0.0224 kJ/g | critical temperature | 500 K | (at STP)
Chemical identifiers
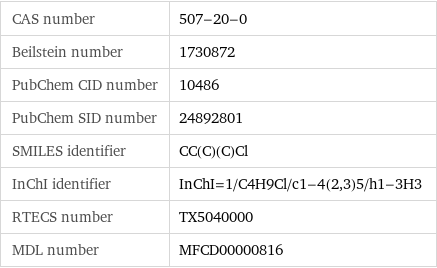
CAS number | 507-20-0 Beilstein number | 1730872 PubChem CID number | 10486 PubChem SID number | 24892801 SMILES identifier | CC(C)(C)Cl InChI identifier | InChI=1/C4H9Cl/c1-4(2, 3)5/h1-3H3 RTECS number | TX5040000 MDL number | MFCD00000816
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 3 NFPA reactivity rating | 0
Safety properties
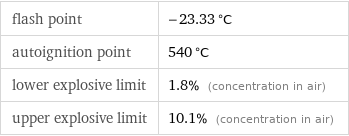
flash point | -23.33 °C autoignition point | 540 °C lower explosive limit | 1.8% (concentration in air) upper explosive limit | 10.1% (concentration in air)

DOT hazard class | 3 DOT numbers | 1127
Toxicity properties
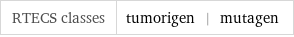
RTECS classes | tumorigen | mutagen