Input interpretation

metallic elements | atomic mass
Summary
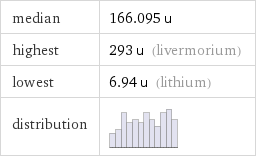
median | 166.095 u highest | 293 u (livermorium) lowest | 6.94 u (lithium) distribution |
Units

Distribution plots
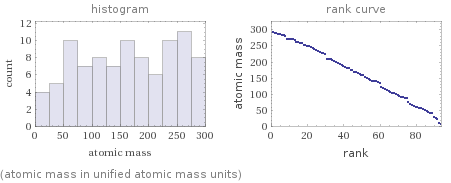
(atomic mass in unified atomic mass units)
Atomic mass rankings
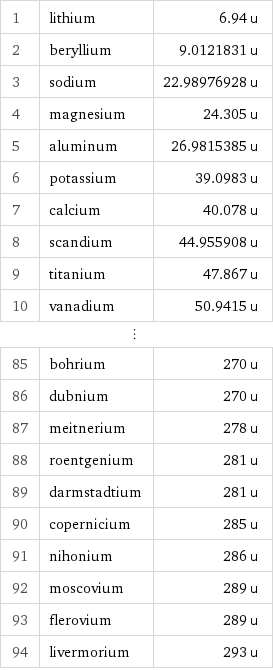
1 | lithium | 6.94 u 2 | beryllium | 9.0121831 u 3 | sodium | 22.98976928 u 4 | magnesium | 24.305 u 5 | aluminum | 26.9815385 u 6 | potassium | 39.0983 u 7 | calcium | 40.078 u 8 | scandium | 44.955908 u 9 | titanium | 47.867 u 10 | vanadium | 50.9415 u ⋮ | | 85 | bohrium | 270 u 86 | dubnium | 270 u 87 | meitnerium | 278 u 88 | roentgenium | 281 u 89 | darmstadtium | 281 u 90 | copernicium | 285 u 91 | nihonium | 286 u 92 | moscovium | 289 u 93 | flerovium | 289 u 94 | livermorium | 293 u
Unit conversions for median atomic mass 166.095 u
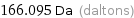
166.095 Da (daltons)
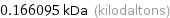
0.166095 kDa (kilodaltons)
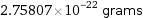
2.75807×10^-22 grams
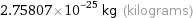
2.75807×10^-25 kg (kilograms)
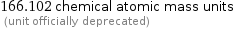
166.102 chemical atomic mass units (unit officially deprecated)
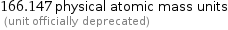
166.147 physical atomic mass units (unit officially deprecated)
Corresponding quantities
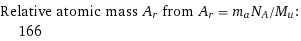
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 166
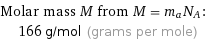
Molar mass M from M = m_aN_A: | 166 g/mol (grams per mole)