Input interpretation

period 2 elements | atomic mass
Summary
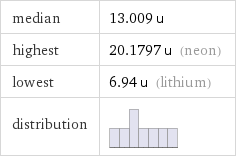
median | 13.009 u highest | 20.1797 u (neon) lowest | 6.94 u (lithium) distribution |
Units

Ranked values
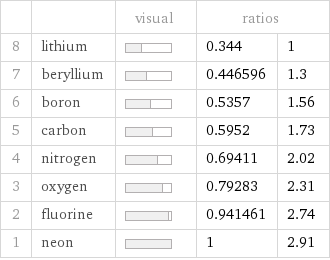
| | visual | ratios | 8 | lithium | | 0.344 | 1 7 | beryllium | | 0.446596 | 1.3 6 | boron | | 0.5357 | 1.56 5 | carbon | | 0.5952 | 1.73 4 | nitrogen | | 0.69411 | 2.02 3 | oxygen | | 0.79283 | 2.31 2 | fluorine | | 0.941461 | 2.74 1 | neon | | 1 | 2.91
Atomic mass rankings
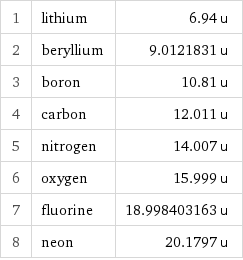
1 | lithium | 6.94 u 2 | beryllium | 9.0121831 u 3 | boron | 10.81 u 4 | carbon | 12.011 u 5 | nitrogen | 14.007 u 6 | oxygen | 15.999 u 7 | fluorine | 18.998403163 u 8 | neon | 20.1797 u
Unit conversions for median atomic mass 13.009 u
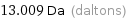
13.009 Da (daltons)
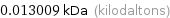
0.013009 kDa (kilodaltons)
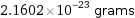
2.1602×10^-23 grams
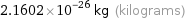
2.1602×10^-26 kg (kilograms)

13.01 chemical atomic mass units (unit officially deprecated)
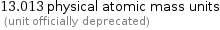
13.013 physical atomic mass units (unit officially deprecated)
Comparison for median atomic mass 13.009 u
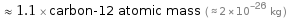
≈ 1.1 × carbon-12 atomic mass ( ≈ 2×10^-26 kg )
Corresponding quantities
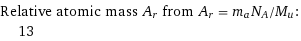
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 13
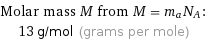
Molar mass M from M = m_aN_A: | 13 g/mol (grams per mole)