Input interpretation

period 4 elements | molar heat of vaporization
Summary
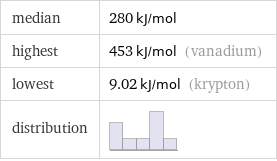
median | 280 kJ/mol highest | 453 kJ/mol (vanadium) lowest | 9.02 kJ/mol (krypton) distribution |
Units

Distribution plots
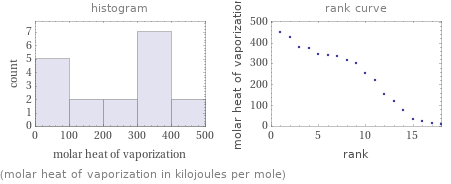
(molar heat of vaporization in kilojoules per mole)
Molar heat of vaporization rankings
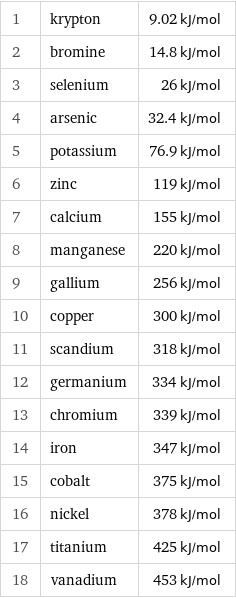
1 | krypton | 9.02 kJ/mol 2 | bromine | 14.8 kJ/mol 3 | selenium | 26 kJ/mol 4 | arsenic | 32.4 kJ/mol 5 | potassium | 76.9 kJ/mol 6 | zinc | 119 kJ/mol 7 | calcium | 155 kJ/mol 8 | manganese | 220 kJ/mol 9 | gallium | 256 kJ/mol 10 | copper | 300 kJ/mol 11 | scandium | 318 kJ/mol 12 | germanium | 334 kJ/mol 13 | chromium | 339 kJ/mol 14 | iron | 347 kJ/mol 15 | cobalt | 375 kJ/mol 16 | nickel | 378 kJ/mol 17 | titanium | 425 kJ/mol 18 | vanadium | 453 kJ/mol
Unit conversions for median molar heat of vaporization 280 kJ/mol
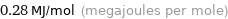
0.28 MJ/mol (megajoules per mole)
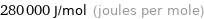
280000 J/mol (joules per mole)
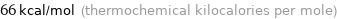
66 kcal/mol (thermochemical kilocalories per mole)
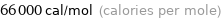
66000 cal/mol (calories per mole)
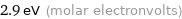
2.9 eV (molar electronvolts)