Input interpretation

aluminum oxide | cerium(IV) fluoride
Chemical names and formulas
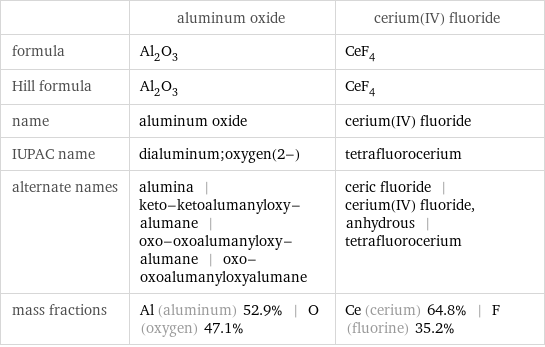
| aluminum oxide | cerium(IV) fluoride formula | Al_2O_3 | CeF_4 Hill formula | Al_2O_3 | CeF_4 name | aluminum oxide | cerium(IV) fluoride IUPAC name | dialuminum;oxygen(2-) | tetrafluorocerium alternate names | alumina | keto-ketoalumanyloxy-alumane | oxo-oxoalumanyloxy-alumane | oxo-oxoalumanyloxyalumane | ceric fluoride | cerium(IV) fluoride, anhydrous | tetrafluorocerium mass fractions | Al (aluminum) 52.9% | O (oxygen) 47.1% | Ce (cerium) 64.8% | F (fluorine) 35.2%
Structure diagrams
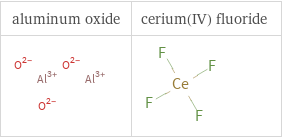
Structure diagrams
Basic properties
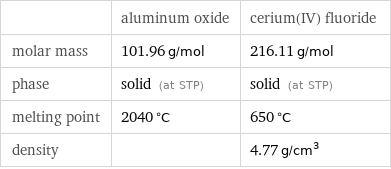
| aluminum oxide | cerium(IV) fluoride molar mass | 101.96 g/mol | 216.11 g/mol phase | solid (at STP) | solid (at STP) melting point | 2040 °C | 650 °C density | | 4.77 g/cm^3
Units

Thermodynamic properties
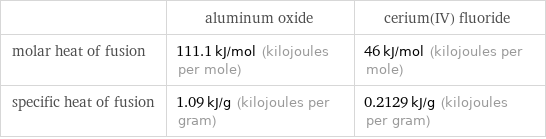
| aluminum oxide | cerium(IV) fluoride molar heat of fusion | 111.1 kJ/mol (kilojoules per mole) | 46 kJ/mol (kilojoules per mole) specific heat of fusion | 1.09 kJ/g (kilojoules per gram) | 0.2129 kJ/g (kilojoules per gram)
Solid properties (at STP)
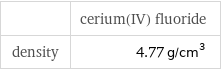
| cerium(IV) fluoride density | 4.77 g/cm^3
Units
